Abstract
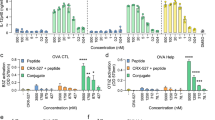
For effective cancer immunotherapy by vaccination, co-delivery of tumour antigens and adjuvants to dendritic cells and subsequent activation of antigen-specific cytotoxic T cells (CTLs) is crucial. In this study, a synthetic long peptide (SLP) harbouring the model CTL epitope SIINFEKL was encapsulated with the TLR3 ligand poly(inosinic-polycytidylic acid) (poly(I:C)) in cationic liposomes consisting of DOTAP and DOPC. The obtained particles were down-sized to about 140 nm (measured by dynamic light scattering) and had a positive zeta-potential of about 26 mV (according to laser Doppler electrophoresis). SLP loading efficiency was about 40% as determined by HPLC. Poly(I:C) loading efficiency was about 50%, as assessed from the fluorescence intensity of fluorescently labelled poly(I:C). Immunogenicity of the liposomal SLP vaccine was evaluated in vitro by its capacity to activate dendritic cells (DCs) and present the processed SLP to SIINFEKL-specific T cells. The effectiveness of the vaccine to activate CD8+ T cells was analysed in vivo after intradermal and subcutaneous immunisation in mice, by measuring antigen-specific T cells in blood and spleens and assessing their functionality by cytokine production and in vivo cytotoxicity. The liposomal formulation efficiently delivered the SLP to DCs in vitro and induced a functional CD8+ T cell immune response in vivo to the CTL epitope present in the SLP. The SLP-specific CD8+ T cell frequency induced by the poly(I:C)-adjuvanted liposomal SLP formulation showed an at least 25 fold increase over the T cell frequency induced by the poly(I:C)-adjuvanted soluble SLP. In conclusion, cationic liposomes loaded with SLP and poly(I:C) have potential as a powerful therapeutic cancer vaccine formulation.








Similar content being viewed by others

References
Melief CJ, van der Burg SH. Immunotherapy of established (pre)malignant disease by synthetic long peptide vaccines. Nat Rev Cancer. 2008;8(5):351–60.
Shanker A, Marincola FM. Cooperativity of adaptive and innate immunity: implications for cancer therapy. Cancer Immunol Immunother. 2011;60(8):1061–74.
Steinman R, Inaba K. Immunogenicity: role of dendritic cells. Bioessays. 1989;10(5):145–52.
Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–96.
Appay V, Douek DC, Price DA. CD8+ T cell efficacy in vaccination and disease. Nat Med. 2008;14(6):623–8.
Vulink A, Radford KJ, Melief C, Hart DN. Dendritic cells in cancer immunotherapy. Adv Cancer Res. 2008;99:363–407.
Inaba K, Young JW, Steinman RM. Direct activation of CD8+ cytotoxic T lymphocytes by dendritic cells. J Exp Med. 1987;166(1):182–94.
Melief CJ. Cancer immunotherapy by dendritic cells. Immunity. 2008;29(3):372–83.
Rosalia RA, Quakkelaar ED, Redeker A, Khan S, Camps M, Drijfhout JW, et al. Dendritic cells process synthetic long peptides better than whole protein, improving antigen presentation and T-cell activation. Eur J Immunol [Research Support, Non-US Gov’t]. 2013;43(10):2554–65.
Bijker MS, van den Eeden SJ, Franken KL, Melief CJ, van der Burg SH, Offringa R. Superior induction of anti-tumor CTL immunity by extended peptide vaccines involves prolonged, DC-focused antigen presentation. Eur J Immunol. 2008;38(4):1033–42.
Kenter GG, Welters MJ, Valentijn AR, Lowik MJ. Berends-van der Meer DM, Vloon AP, et al. Phase I immunotherapeutic trial with long peptides spanning the E6 and E7 sequences of high-risk human papillomavirus 16 in end-stage cervical cancer patients shows low toxicity and robust immunogenicity. Clin Cancer Res. 2008;14(1):169–77.
Aucouturier J, Ascarateil S, Dupuis L. The use of oil adjuvants in therapeutic vaccines. Vaccine. 2006;24(2):S2-44–5.
Cai S, Yang Q, Bagby TR, Forrest ML. Lymphatic drug delivery using engineered liposomes and solid lipid nanoparticles. Adv Drug Deliv Rev. 2011;63(10–11):901–8.
Zaks K, Jordan M, Guth A, Sellins K, Kedl R, Izzo A, et al. Efficient immunization and cross-priming by vaccine adjuvants containing TLR3 or TLR9 agonists complexed to cationic liposomes. J Immunol. 2006;176(12):7335–45.
Nordly P, Rose F, Christensen D, Nielsen HM, Andersen P, Agger EM, et al. Immunity by formulation design: induction of high CD8+ T-cell responses by poly(I:C) incorporated into the CAF01 adjuvant via a double emulsion method. J Control Release. 2011;150(3):307–17.
Hansen J, Lindenstrom T, Lindberg-Levin J, Aagaard C, Andersen P, Agger EM. CAF05: cationic liposomes that incorporate synthetic cord factor and poly(I:C) induce CTL immunity and reduce tumor burden in mice. Cancer Immunol Immunother. 2012;61(6):893–903.
Gregoriadis G. The immunological adjuvant and vaccine carrier properties of liposomes. J Drug Target. 1994;2(5):351–6.
Al-Jamal WT, Kostarelos K. Liposomes: from a clinically established drug delivery system to a nanoparticle platform for theranostic nanomedicine. Acc Chem Res. 2011;44(10):1094–104.
Hubbell JA, Thomas SN, Swartz MA. Materials engineering for immunomodulation. Nature. 2009;462(7272):449–60.
Watson DS, Endsley AN, Huang L. Design considerations for liposomal vaccines: influence of formulation parameters on antibody and cell-mediated immune responses to liposome associated antigens. Vaccine. 2012;30(13):2256–72.
Bachmann MF, Jennings GT. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol. 2010;10(11):787–96.
Joshi MD, Unger WJ, Storm G, van Kooyk Y, Mastrobattista E. Targeting tumor antigens to dendritic cells using particulate carriers. J Control Release. 2012;161(1):25–37.
Ossendorp F, Mengede E, Camps M, Filius R, Melief CJ. Specific T helper cell requirement for optimal induction of cytotoxic T lymphocytes against major histocompatibility complex class II negative tumors. J Exp Med. 1998;187(5):693–702.
Gregoriadis G, Bacon A, Caparros-Wanderley W, McCormack B. Plasmid DNA vaccines: entrapment into liposomes by dehydration-rehydration. Methods Enzymol. 2003;367:70–80.
Silva AL, Rosalia RA, Sazak A, Carstens MG, Ossendorp F, Oostendorp J, et al. Optimization of encapsulation of a synthetic long peptide in PLGA nanoparticles: low-burst release is crucial for efficient CD8+ T cell activation. Eur J Pharm Biopharm. 2013;83(3):338–45.
Winzler C, Rovere P, Rescigno M, Granucci F, Penna G, Adorini L, et al. Maturation stages of mouse dendritic cells in growth factor-dependent long-term cultures. J Exp Med. 1997;185(2):317–28.
Sanderson S, Shastri N. LacZ inducible, antigen/MHC-specific T cell hybrids. Int Immunol. 1994;6(3):369–76.
Bal SM, Hortensius S, Ding Z, Jiskoot W, Bouwstra JA. Co-encapsulation of antigen and Toll-like receptor ligand in cationic liposomes affects the quality of the immune response in mice after intradermal vaccination. Vaccine. 2011;29(5):1045–52.
Colonna C, Conti B, Genta I, Alpar OH. Non-viral dried powders for respiratory gene delivery prepared by cationic and chitosan loaded liposomes. Int J Pharm. 2008;364(1):108–18.
Voehringer D, Koschella M, Pircher H. Lack of proliferative capacity of human effector and memory T cells expressing killer cell lectinlike receptor G1 (KLRG1). Blood. 2002;100(10):3698–702.
Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–63.
Ma Y, Zhuang Y, Xie X, Wang C, Wang F, Zhou D, et al. The role of surface charge density in cationic liposome-promoted dendritic cell maturation and vaccine-induced immune responses. Nanoscale. 2011;3(5):2307–14.
Chen W, Yan W, Huang L. A simple but effective cancer vaccine consisting of an antigen and a cationic lipid. Cancer Immunol Immunother. 2008;57(4):517–30.
Yan W, Chen W, Huang L. Reactive oxygen species play a central role in the activity of cationic liposome based cancer vaccine. J Control Release. 2008;130(1):22–8.
Campbell RB, Balasubramanian SV, Straubinger RM. Phospholipid-cationic lipid interactions: influences on membrane and vesicle properties. Biochim Biophys Acta. 2001;1512(1):27–39.
de Jong S, Chikh G, Sekirov L, Raney S, Semple S, Klimuk S, et al. Encapsulation in liposomal nanoparticles enhances the immunostimulatory, adjuvant and anti-tumor activity of subcutaneously administered CpG ODN. Cancer Immunol Immunother. 2007;56(8):1251–64.
Lesterhuis WJ, de Vries IJ, Schreibelt G, Lambeck AJ, Aarntzen EH, Jacobs JF, et al. Route of administration modulates the induction of dendritic cell vaccine-induced antigen-specific T cells in advanced melanoma patients. Clin Cancer Res. 2011;17(17):5725–35.
Korsholm KS, Hansen J, Karlsen K, Filskov J, Mikkelsen M, Lindenstrom T, et al. Induction of CD8+ T-cell responses against subunit antigens by the novel cationic liposomal CAF09 adjuvant. Vaccine. 2014;32(31):3927–35.
Aguilar JC, Rodriguez EG. Vaccine adjuvants revisited. Vaccine. 2007;25(19):3752–62.
Ludewig B, Barchiesi F, Pericin M, Zinkernagel RM, Hengartner H, Schwendener RA. In vivo antigen loading and activation of dendritic cells via a liposomal peptide vaccine mediates protective antiviral and anti-tumour immunity. Vaccine. 2000;19(1):23–32.
Zhang J, Tian H, Li C, Cheng L, Zhang S, Zhang X, et al. Antitumor effects obtained by autologous Lewis lung cancer cell vaccine engineered to secrete mouse interleukin 27 by means of cationic liposome. Mol Immunol. 2013;55(3–4):264–74.
van Duikeren S, Fransen MF, Redeker A, Wieles B, Platenburg G, Krebber WJ, et al. Vaccine-induced effector-memory CD8+ T cell responses predict therapeutic efficacy against tumors. J Immunol. 2012;189(7):3397–403.
van Duikeren S, Arens R. Predicting the efficacy of cancer vaccines by evaluating T-cell responses. Oncoimmunology. 2013;2(1):e22616.
Wang C, Zhuang Y, Zhang Y, Luo Z, Gao N, Li P, et al. Toll-like receptor 3 agonist complexed with cationic liposome augments vaccine-elicited antitumor immunity by enhancing TLR3-IRF3 signaling and type I interferons in dendritic cells. Vaccine. 2012;30(32):4790–9.
Reimer T, Brcic M, Schweizer M, Jungi TW. Poly(I:C) and LPS induce distinct IRF3 and NF-kappaB signaling during type-I IFN and TNF responses in human macrophages. J Leukoc Biol. 2008;83(5):1249–57.
Gitlin L, Barchet W, Gilfillan S, Cella M, Beutler B, Flavell RA, et al. Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc Natl Acad Sci U S A. 2006;103(22):8459–64.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Guest Editor: Aliasger Salem
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 448 kb)
Rights and permissions
About this article
Cite this article
Varypataki, E.M., van der Maaden, K., Bouwstra, J. et al. Cationic Liposomes Loaded with a Synthetic Long Peptide and Poly(I:C): a Defined Adjuvanted Vaccine for Induction of Antigen-Specific T Cell Cytotoxicity. AAPS J 17, 216–226 (2015). https://doi.org/10.1208/s12248-014-9686-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-014-9686-4