Abstract
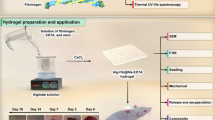
Fabrication of 3D composite scaffolds was carried out by lyophilization of variable concentrations of collagen and chitosan gel solutions. Fibrinogen and thrombin aerosol were deposited over the surface of scaffolds to enhance hemostasis and wound healing. Composite scaffolds were characterized using differential scanning calorimetry, thermogravimetric analysis, and Fourier-transform infrared spectrophotometer to ascertain the aerosol deposition and stability. Scanning electron microscope showed multilayered porosity with pore size of ~30 μm and mushroom-like fibril growth of aerosol. A detailed investigation by surface plasmon resonance confirmed higher binding affinity of collagen toward the human blood platelets and erythrocytes in comparison to chitosan and was found to increase with the increase in blood cell concentration from 480.8 to 886.4 RU for erythrocytes. Scaffolds showed higher binding response for platelets than erythrocytes, while fibrinogen and thrombin showed no or limited interaction. Highest blood sorption of 83 ± 4% was observed in case of aerosol deposited scaffolds. Aerosol deposited scaffolds showed minimum clotting time of 20 ± 3 s and bleeding time of 38 ± 4 s, which was significantly lower compared to the scaffolds without aerosol treatment. Aerosol deposited composite scaffolds with 2:1 concentration of chitosan/collagen showed complete wound contraction by day 14, while 50% was observed in case of the control group. In vivo studies revealed that chitosan had a crucial role in the inflammatory phase, while collagen played an important role in the proliferation and maturation phase. The present study suggests that the fabricated 3D composite scaffolds with bioactive moieties may be a potential candidate for enhanced hemostasis and wound healing applications.
Graphical abstract









Similar content being viewed by others
Change history
08 June 2022
A Correction to this paper has been published: https://doi.org/10.1208/s12249-022-02317-6
References
Valdez C, Sarani B, Young H, Amdur R, Dunne J, Chawla LS. Timing of death after traumatic injury—a contemporary assessment of the temporal distribution of death. J Surg Res. 2016;200(2):604–9.
Kunio NR, Riha GM, Watson KM, Differding JA, Schreiber MA, Watters JM. Chitosan based advanced haemostatic dressing is associated with decreased blood loss in a swine uncontrolled haemorrhage model. Am J Surg. 2013;205(5):505–10.
Seyednejad H, Imani M, Jamieson T, Seifalian AM, Brown EE, Laborie MP, et al. Topical haemostatic agents. Br J Surg. 2008;95(10):1197–225.
Peng T. Biomaterials for hemorrhage control. Trends Biomater Artif Organs. 2010;24(1):27–68.
Gousia Sheikh QM, Majeed S, Hassan I. Comparison of levels of serum copper, zinc, albumin, globulin and alkaline phosphatase in psoriatic patients and controls: a hospital based case control study. Indian Dermatol Online J. 2015;6(2):81.
Björk J, Hugli TE, Smedegård G. Microvascular effects of anaphylatoxins C3a and C5a. J Immunol. 1985;134(2):1115–9.
Clark RA, editor. The molecular and cellular biology of wound repair: Springer Science & Business Media; 2013.
Leibovich SJ, Ross R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol. 1975;78(1):71.
Gómez-Guillén MC, Giménez B, López-Caballero MA, Montero MP. Functional and bioactive properties of collagen and gelatin from alternative sources: a review. Food Hydrocoll. 2011;25(8):1813–27.
Morishige H, Sugahara T, Nishimoto S, Muranaka A, Ohno F, Shiraishi R, et al. Immunostimulatory effects of collagen from jellyfish in vivo. Cytotechnology. 2011;63(5):481–92.
Fan J, Zhuang Y, Li B. Effects of collagen and collagen hydrolysate from jellyfish umbrella on histological and immunity changes of mice photoaging. Nutrients. 2013;5(1):223–33.
Alemán A, Gómez-Guillén MC, Montero P. Identification of ace-inhibitory peptides from squid skin collagen after in vitro gastrointestinal digestion. Food Res Int. 2013;54(1):790–5.
Barzideh Z, Latiff AA, Gan CY, Abedin M, Alias AK. ACE inhibitory and antioxidant activities of collagen hydrolysates from the ribbon jellyfish (Chrysaora sp.). Food Technol Biotechnol. 2014;52(4):495–504.
Zhuang Y, Sun L, Zhang Y, Liu G. Antihypertensive effect of long-term oral administration of jellyfish (Rhopilema esculentum) collagen peptides on renovascular hypertension. Mar Drugs. 2012;10(2):417–26.
Ennaas N, Hammami R, Gomaa A, Bédard F, Biron É, Subirade M, et al. Collagencin, an antibacterial peptide from fish collagen: activity, structure and interaction dynamics with membrane. Biochem Biophys Res Commun. 2016;473(2):642–7.
Ennaas N, Hammami R, Beaulieu L, Fliss I. Purification and characterization of four antibacterial peptides from protamex hydrolysate of Atlantic mackerel (Scomber scombrus) by-products. Biochem Biophys Res Commun. 2015;462(3):195–200.
Chi CF, Cao ZH, Wang B, Hu FY, Li ZR, Zhang B. Antioxidant and functional properties of collagen hydrolysates from Spanish mackerel skin as influenced by average molecular weight. Molecules. 2014;19(8):11211–30.
Asserin J, Lati E, Shioya T, Prawitt J. The effect of oral collagen peptide supplementation on skin moisture and the dermal collagen network: evidence from an ex vivo model and randomized, placebo-controlled clinical trials. J Cosmet Dermatol. 2015;14(4):291–301.
Addad S, Exposito JY, Faye C, Ricard-Blum S, Lethias C. Isolation, characterization and biological evaluation of jellyfish collagen for use in biomedical applications. Mar Drugs. 2011;9(6):967–83.
Lim CK, Halim AS. Biomedical-grade chitosan in wound management and its biocompatibility in vitro. IntechOpen Limited, London. Biopolymers. 2010:19–33.
Venkatesan J, Vinodhini PA, Sudha PN, Kim SK. Chitin and chitosan composites for bone tissue regeneration. Adv Food Nutr Res. 2014;73:59–81.
Ahmed S, Ikram S. Chitosan based scaffolds and their applications in wound healing. Achiev Life Sci. 2016;10(1):27–37.
Liu L, Wen H, Rao Z, Zhu C, Liu M, Min L, et al. Preparation and characterization of chitosan-collagen peptide/oxidized konjac glucomannan hydrogel. Int J Biol Macromol. 2018;108:376–82.
Zhu X, Zhou X, Yi J, Tong J, Wu H, Fan L. Preparation and biological activity of quaternized carboxymethyl chitosan conjugated with collagen peptide. Int J Biol Macromol. 2014;70:300–5.
Zhang C, Yang X, Hu W, Han X, Fan L, Tao S. Preparation and characterization of carboxymethyl chitosan/collagen peptide/oxidized konjac composite hydrogel. Int J Biol Macromol. 2020;149:31–40.
De Moerloose P, Casini A, Neerman-Arbez M. Congenital fibrinogen disorders: an update. Semin Thromb Hemost. 2013;39(06):585–995.
Jiang Y, Doolittle RF. The evolution of vertebrate blood coagulation as viewed from a comparison of puffer fish and sea squirt genomes. Proc Natl Acad Sci. 2003;100(13):7527–32.
Rydzak J, Kaczmarek R, Czerwinski M, Lukasiewicz J, Tyborowska J. PLoS One. 2015;10(1):e0115437.
de Lima JM, Sarmento RR, de Souza JR, Brayner FA, Feitosa APS, Padilha R, et al. Evaluation of hemagglutination activity of chitosan nanoparticles using human erythrocytes. Biomed Res Int. 2015;247965:6. https://doi.org/10.1155/2015/247965.
García-Manzano A, González-Llaven J, Lemini C, Rubio-Póo C. Standardization of rat blood clotting tests with reagents used for humans. Proc West Pharmacol Soc. 2001;44:153–155.
Quinn JG, O’Neill S, Doyle A, McAtamney C, Diamond D, MacCraith BD, et al. Development and application of surface plasmon resonance-based biosensors for the detection of cell–ligand interactions. Anal Biochem. 2000;281(2):135–43.
Stavitsky AB. Micromethods for the study of proteins and antibodies. I. Procedure and general applications of hemagglutination and hemagglutination-inhibition reactions with tannic acid and protein-treated red blood cells. J Immunol. 1954;72(5):360–7.
Bettini R, Romani AA, Morganti MM, Borghetti AF. Physicochemical and cell adhesion properties of chitosan films prepared from sugar and phosphate containing solutions. Eur J Pharm Biopharm. 2008;68:74–81.
Vidinský B, Gál P, Toporcer T, Longauer F, Lenhardt Ľ, Bobrov N, et al. Histological study of the first seven days of skin wound healing in rats. Acta Vet Brno. 2006;75:197–202.
Acknowledgments
The above research work was financially supported under the project TD/INM-321 by Institute of Nuclear Medicine and Allied Sciences, Defence Research and Development Organization (INMAS-DRDO), New Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tripathi, D., Sharma, A., Tyagi, P. et al. Fabrication of Three-Dimensional Bioactive Composite Scaffolds for Hemostasis and Wound Healing. AAPS PharmSciTech 22, 138 (2021). https://doi.org/10.1208/s12249-021-02010-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-021-02010-0