Abstract
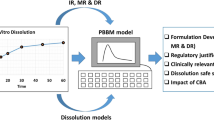
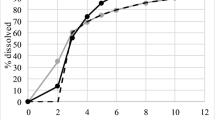
The objective of the study was to predict pharmacokinetic (PK) and pharmacodynamic (PD) parameters of matrix-based modified release (MR) drug product of vildagliptin. Physiologically based biopharmaceutics modeling (PBBM) was developed using GastroPlus™ based on the available data including immediate-release (IR) drug product of vildagliptin. In vitro–in vivo correlation (IVIVC) was developed using mechanistic deconvolution to predict plasma concentration–time profile and PK parameters for a MR drug product planned for clinical use. Both methods, i.e., PBBM and IVIVC, were compared for the predicted PK parameters. Integration of DDDPlus™ and GastroPlus™ modeling was performed to explore clinically relevant dissolution specifications for vildagliptin MR tablets. The bioequivalence (BE) between batches with different dissolution specifications was evaluated using virtual clinical trials. The PD effect of dipeptidyl peptidase-IV (DPP-IV) inhibition was simulated utilizing PDPlus™ model in GastroPlus™. The results indicated that IVIVC best correlated the simulated PK parameters with those observed with the clinical study. The outcomes highlight the importance of integration of in vitro and in silico tools towards predictability of PK and PD parameters for a MR drug product. However, the post absorptive phase was found to be more dependent on the demographics of the healthy subjects.














Similar content being viewed by others
References
Shoback DG, Gardner D, eds. Chapter 17. Greenspan’s basic & clinical endocrinology (9th ed.). 2011. New York: McGraw-Hill Medical. ISBN 978–0–07–162243–1.
Sunkara Gangadhar, Sabo Ron, et al. Dose proportionality and the effect of food on vildagliptin, a novel dipeptidyl peptidase IV inhibitor, healthy volunteers. J Clin Pharmacol. 2007;47:1152–8.
He Y-L, Sadler BM, et al. The absolute oral bioavailability and population-based pharmacokinetic modelling of a novel dipeptidylpeptidase-IV inhibitor, vildagliptin, in healthy volunteers. Clin Pharmacokinet. 2007;46(9):787–802.
He Handan, Tran Phi, et al. Absorption, metabolism, and excretion of [14C] vildagliptin, a novel dipeptidyl peptidase 4 inhibitor, in humans. Drug Metabolism and Disposition. 2009;37(3):536–44.
The use of physiologically based pharmacokinetic analyses – biopharmaceutics applications for oral drug product development, manufacturing changes, and controls. Guidance for Industry. Draft Guidance. U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER), October 2020
Guideline on reporting of physiologically based pharmacokinetic (PBPK) modeling and simulation. Committee for Medicinal Products for Human Use (CHMP), European Medicines Agency (EMA), December 2018.
Extended release oral dosage forms: development, evaluation and application of in vitro/in vivo correlations. Guidance for Industry; U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER), September 1997
Al-Tabakha Moawia M, Alomer Muaed J. In vitro dissolution and in silico modeling shortcuts in bioequivalence testing. Pharmaceutics. 2020;12:45.
McAllister M, Flanagan T, Boon K, et al. Developing relevant dissolution specifications for oral drug products - industrial and regulatory perspectives. Pharmaceutics. 2019;12(1):19.
Sjögren E, Thörn H, Tannergren C. In silico modeling of gastrointestinal drug absorption: predictive performance of three physiologically based absorption models. Mol Pharmaceutics. 2016;13:1763–78.
May Almukainzi, Arthur Okumu, Hai Wei and Raimar Löbenberg. Simulation of in vitro dissolution behavior using DDDPlus™. AAPS PharmSciTech, Vol. 16, No. 1, February 2015
GastroPlus™ User Manual for Version 9.7. Lancaster, CA: Simulations Plus, 2019
DDDPlus® User Manual for Version 6.0. Lancaster, CA: Simulations Plus, 2018
McAllister M, Flanagan T, Boon K, Pepin X, Tistaert C, Jamei M, Abend A, Kotzagiorgis E, Mackie C. Developing clinically relevant dissolution specifications for oral drug products - industrial and regulatory perspectives. Pharmaceutics. 2020;12:19.
Food and Drug Administration. Guidance for Industry: Extended Release Oral Dosage Forms: Development, Evaluation and Application of In Vitro/In Vivo Correlations. 1997.
European Medicines Agency. Guideline on the pharmacokinetic and clinical evaluation of modified release dosage forms. 2014
DR Mould; RN Upton; Basic Concepts in Population Modeling, Simulation, and Model-Based Drug Development; Pharmacometrics & Systems Pharmacology (2012) 1, e6.
Deurenberg P, Deurenberg-Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass Index/body fat percent relationship. Obes Rev. 2002;3(3):141–6.
Zhang X, Lionberger RA, Davit BM, Yu LX. Utility of physiologically based absorption modeling in implementing quality by design in drug development. AAPS J. 2011;13:59–71.
Miller NA, Graves RH, Edwards CD, et al. Physiologically based pharmacokinetic modelling of inhaled nemiralisib: mechanistic components for pulmonary absorption, systemic distribution, and oral absorption. Clin Pharmacokinet. 2021.
Yang F, et al. Prediction of a therapeutic dose for buagafuran, a potent anxiolytic agent by physiologically based pharmacokinetic/pharmacodynamic modeling starting from pharmacokinetics in rats and human. Front Pharmacol. 2017;8:683.
Basu S, et al. Physiologically based pharmacokinetic modeling to evaluate formulation factors influencing bioequivalence of metoprolol extended-release products. J Clin Pharmacol. 2019;59(9):1252–63.
Babiskin A, Zhang X. Application of physiologically based absorption modeling for amphetamine salts drug products in generic drug evaluation. J Pharm Sci. 2015;104:3710–3180.
Acknowledgements
The authors hereby gratefully acknowledge the efforts from the Novartis associates working in analytical research and development (ARD) for their support in analytical activities, associates from manufacturing plant for supporting manufacturing activities, Biopharmaceutics Expert Group (BPEG), and all those who have played their role during the entire Galvus OD development journey. We thank all the investigators, study coordinators, subjects who participated in the study, and local laboratory and health facility staff present at the clinical study and testing sites. The authors are also grateful to Novartis Global Program Team (GPT) for their scientific guidance and support provided during the development of the drug product.
Funding
The study presented in this article was fully funded by Novartis Healthcare Pvt. Ltd. India.
Author information
Authors and Affiliations
Contributions
Muzaffaruddin Ahmed Madny: conceptualization, data curation, methodology, software, investigation, writing—original draft, formal analysis.
Pandurang Deshpande: resources, data curation, supervision, writing—review and editing.
Venkat Tumuluri: resources, writing—review and editing, project administration.
Parag Borde: conceptualization, supervision, project administration, writing—review and editing.
Ramachandra Sangana: resources, writing—review and editing, validation, formal analysis.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Madny, M.A., Deshpande, P., Tumuluri, V. et al. Physiologically Based Biopharmaceutics Model of Vildagliptin Modified Release (MR) Tablets to Predict In Vivo Performance and Establish Clinically Relevant Dissolution Specifications. AAPS PharmSciTech 23, 108 (2022). https://doi.org/10.1208/s12249-022-02264-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-022-02264-2