Abstract
Objective
The aim of this study was to examine the survival effect of adjuvant therapy in stage II–III endometrial cancer based on peritoneal cytology results.
Methods
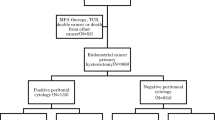
The National Cancer Institute’s Surveillance, Epidemiology, and End Results Program was retrospectively queried to examine 7467 women with stage II–III endometrial cancer who underwent hysterectomy, and with available peritoneal cytology results, from 2010 to 2016. A Cox proportional hazard regression model was fitted to assess the association between adjuvant therapy and all-cause mortality stratified by peritoneal cytology results.
Results
Malignant peritoneal cytology was reported in 1662 (22.3%) women and was associated with non-endometrioid histology, higher tumor stage, and nodal metastasis (p < 0.05). In a propensity score-weighted model, malignant peritoneal cytology was associated with increased all-cause mortality compared with negative peritoneal cytology (hazard ratio 1.35, 95% confidence interval 1.23–1.48). Adjuvant therapy types varied based on histology and peritoneal cytology results. In non-endometrioid histology, the combination of chemotherapy and whole pelvic radiotherapy (WPRT) was associated with improved overall survival compared with chemotherapy or WPRT alone irrespective of the peritoneal cytology results (p < 0.05). The combination of chemotherapy and WPRT was also associated with improved overall survival in women with endometrioid histology and malignant peritoneal cytology (p = 0.026). Women with endometrioid histology and negative peritoneal cytology represented the most common subpopulation (46.5%), and overall survival was similar regardless of which of the three adjuvant therapy modalities was used (p = 0.319).
Conclusions
Malignant peritoneal cytology is prevalent and prognostic in stage II–III endometrial cancer. This study found that the surgeon’s choice and benefit of adjuvant therapy for women with stage II–III endometrial cancer differed depending on the status of peritoneal cytology.





Similar content being viewed by others
Data Availability
The data that support the findings of this study are openly available in the National Cancer Institute’s SEER program (http://seer.cancer.gov/).
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108.
Wright JD, Barrena Medel NI, Sehouli J, Fujiwara K, Herzog TJ. Contemporary management of endometrial cancer. Lancet. 2012;379(9823):1352–60.
Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E. Endometrial cancer. Lancet. 2016;387(10023):1094–108.
Uterine. Neoplasms. NCCN Clinical Practice Guidelines in Oncology. National Comprehensive Cancer Network. https://www.nccn.org/professionals/physician_gls/. Accessed 23 Jul 2020.
Yamagami W, Mikami M, Nagase S, et al. Japan Society of Gynecologic Oncology 2018 guidelines for treatment of uterine body neoplasms. J Gynecol Oncol. 2020;31(1):e18.
Colombo N, Creutzberg C, Amant F, et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: diagnosis, treatment and follow-up. Ann Oncol. 2016;27(1):16–41.
Matsuo K, Matsuzaki S, Nusbaum DJ, et al. Malignant peritoneal cytology and decreased survival of women with stage I endometrioid endometrial cancer. Eur J Cancer. 2020;133:33–46.
Matsuo K, Yabuno A, Hom MS, et al. Significance of abnormal peritoneal cytology on survival of women with stage I–II endometrioid endometrial cancer. Gynecol Oncol. 2018;149(2):301–9.
Tate K, Yoshida H, Ishikawa M, et al. Prognostic factors for patients with early-stage uterine serous carcinoma without adjuvant therapy. J Gynecol Oncol. 2018;29(3):e34.
Seagle BL, Alexander AL, Lantsman T, Shahabi S. Prognosis and treatment of positive peritoneal cytology in early endometrial cancer: matched cohort analyses from the National Cancer Database. Am J Obstet Gynecol. 2018;218(3):329.e321-329.e315.
Haltia UM, Bützow R, Leminen A, Loukovaara M. FIGO 1988 versus 2009 staging for endometrial carcinoma: a comparative study on prediction of survival and stage distribution according to histologic subtype. J Gynecol Oncol. 2014;25(1):30–5.
Garg G, Gao F, Wright JD, Hagemann AR, Mutch DG, Powell MA. Positive peritoneal cytology is an independent risk-factor in early stage endometrial cancer. Gynecol Oncol. 2013;128(1):77–82.
Bansal S, Buck AM, Herzog TJ, Deutsch I, Burke WM, Wright JD. Stage IIIA endometrial carcinoma: outcome and predictors of survival. Obstet Gynecol. 2009;114(1):100–5.
Havrilesky LJ, Cragun JM, Calingaert B, et al. The prognostic significance of positive peritoneal cytology and adnexal/serosal metastasis in stage IIIA endometrial cancer. Gynecol Oncol. 2007;104(2):401–5.
Saga Y, Imai M, Jobo T, et al. Is peritoneal cytology a prognostic factor of endometrial cancer confined to the uterus? Gynecol Oncol. 2006;103(1):277–80.
Matsuo K, Nusbaum DJ, Matsuzaki S, et al. Malignant peritoneal cytology and increased mortality risk in stage I non-endometrioid endometrial cancer. Gynecol Oncol. 2020;159(1):43–51.
Kahramanoglu I, Meydanli MM, Taranenka S, et al. SATEN III-splitting adjuvant treatment of stage III ENdometrial cancers: an international, multicenter study. Int J Gynecol Cancer. 2019;29(8):1271–9.
Milgrom SA, Kollmeier MA, Abu-Rustum NR, O’Cearbhaill RE, Barakat RR, Alektiar KM. Quantifying the risk of recurrence and death in stage III (FIGO 2009) endometrial cancer. Gynecol Oncol. 2014;134(2):297–301.
Kuku S, Williams M, McCormack M. Adjuvant therapy in stage III endometrial cancer: treatment outcomes and survival. A single-institution retrospective study. Int J Gynecol Cancer. 2013;23(6):1056–64.
National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program. http://seer.cancer.gov/. Accessed 23 Jul 2020.
Zippin C, Lum D, Hankey BF. Completeness of hospital cancer case reporting from the SEER Program of the National Cancer Institute. Cancer. 1995;76(11):2343–50.
National Cancer Registrars Association. http://www.ncra-usa.org/. Accessed 23 Jul 2020.
CorpusCarcinoma. CS Site-Specific Factor 2 Peritoneal Cytology. http://web2.facs.org/cstage0205/corpuscarcinoma/CorpusCarcinoma_kpo.html. Accessed 23 Jul 2020.
Jamison PM, Altekruse SF, Chang JT, et al. Site-specific factors for cancer of the corpus uteri from SEER registries: collaborative stage data collection system, version 1 and version 2. Cancer. 2014;120(Suppl 23):3836–45.
International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3). World Health Organization. Available at: https://www.who.int/classifications/icd/adaptations/oncology/en/. Accessed 23 Jul 2020.
American. Joint Committee on Cancer. https://cancerstaging.org/Pages/default.aspx. Accessed 23 Jul 2020.
Whitney CW, Spirtos N. Gynecologic Oncology Group surgical procedures manual. Philadelphia, PA: Gynecologic Oncology Group; 2009.
Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661–79.
Cohen J. Statistical power analysis for the behavioral sciences. 2nd edn. Hillsdale, NJ: Erlbaum; 1988.
Pinto D, Chandra A, Crothers BA, Kurtycz DFI, Schmitt F. The international system for reporting serous fluid cytopathology-diagnostic categories and clinical management. J Am Soc Cytopathol. 2020;9(6):469–77.
Pavlou M, Ambler G, Seaman S, De Iorio M, Omar RZ. Review and evaluation of penalised regression methods for risk prediction in low-dimensional data with few events. Stat Med. 2016;35(7):1159–77.
National. Cancer Institute. Joinpoint trend analysis software. https://surveillance.cancer.gov/joinpoint/. Accessed 19 Jul 2020.
Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133(6):601–9.
von Elm E, Altman DG, Egger M, et al. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806–8.
Mayo-de-Las-Casas C, Velasco A, Sanchez D, et al. Detection of somatic mutations in peritoneal lavages and plasma of endometrial cancer patients: a proof-of-concept study. Int J Cancer. 2020;147(1):277–84.
Chang YN, Zhang Y, Wang YJ, Wang LP, Duan H. Effect of hysteroscopy on the peritoneal dissemination of endometrial cancer cells: a meta-analysis. Fertil Steril. 2011;96(4):957–61.
Padilla-Iserte P, Lago V, Tauste C, et al. Impact of uterine manipulator on oncological outcome in endometrial cancer surgery. Am J Obstet Gynecol. 2021;224(1):65 e61-65 e11.
de Boer SM, Powell ME, Mileshkin L, et al. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19(3):295–309.
de Boer SM, Powell ME, Mileshkin L, et al. Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial. Lancet Oncol. 2019;20(9):1273–85.
Matei D, Filiaci V, Randall ME, et al. Adjuvant chemotherapy plus radiation for locally advanced endometrial cancer. N Engl J Med. 2019;380(24):2317–26.
van Weelden WJ, Reijnen C, Eggink FA, et al. Impact of different adjuvant treatment approaches on survival in stage III endometrial cancer: a population-based study. Eur J Cancer. 2020;133:104–11.
Syeda S, Chen L, Hou JY, et al. Chemotherapy, radiation, or combination therapy for stage III uterine cancer. Obstet Gynecol. 2019;134(1):17–29.
Felix AS, Brinton LA, McMeekin DS, et al. Relationships of tubal ligation to endometrial carcinoma stage and mortality in the NRG Oncology/Gynecologic Oncology Group 210 Trial. J Natl Cancer Inst. 2015;107(9):djv158.
de Boer SM, Wortman BG, Bosse T, et al. Clinical consequences of upfront pathology review in the randomised PORTEC-3 trial for high-risk endometrial cancer. Ann Oncol. 2018;29(2):424–30.
Wright JD, Cham S, Chen L, et al. Utilization of sentinel lymph node biopsy for uterine cancer. Am J Obstet Gynecol. 2017;216(6):594 e591-594 e513.
Matsuo K, Matsuzaki S, Roman LD, Klar M, Wright JD. Proposal of an endometrial cancer staging schema with stage-specific incorporation of malignant peritoneal cytology. Am J Obstet Gynecol. 2021;224(3):319–21.
Funding
Koji Matsuo has received funding support from Ensign Endowment for Gynecologic Cancer Research. The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
Koji Matsuo designed the study, initiated the collaborations, cleaned and analyzed the data, created the figures and tables, interpreted the results, and drafted and revised the manuscript with others. He is also the corresponding author of this study. David J. Nusbaum contributed to the analysis, interpreted the results, and revised the manuscript. Shinya Matsuzaki contributed to the literature overview and intellectual inputs, interpreted the results, and edited the manuscript. Lynda D. Roman and Jason D. Wright supervised the study and revised the manuscript. Philipp Harter reviewed the results and revised the manuscript. Maximilian Klar contributed to the study concept and design, instructed the analytic approach, interpreted the results, and revised the manuscript.
Corresponding author
Ethics declarations
Disclosure
The following disclosures are declared, outside the submitted work: Jason D. Wright is a consultant for Clovis Oncology and has undertaken research funding for Merck. Lynda D. Roman is a consultant for Quantgene. Koji Matsuo has received honorarium from Chugai, textbook editorial expenses from Springer, and investigator meeting attendance expenses from VBL therapeutics. Shinya Matsuzaki has received research funding from MSD. Philipp Harter has received personal fees, non-financial support, honoraria for lectures, advisory board fees, and travel support from AstraZeneca and Roche; non-financial support and travel support from Medac; personal fees, honoraria for lectures, and advisory board fees from Tesaro and PharmaMar; personal fees and honoraria for advisory board participation from Clovis and Lilly; personal fees and honoraria for lectures from Stryker; and personal fees and honoraria for advisory board participation from Immunogen. Maximilian Klar has received advisory board fees from Tesaro and GSK. David J. Nusbaum has no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Matsuo, K., Matsuzaki, S., Nusbaum, D.J. et al. Association Between Adjuvant Therapy and Survival in Stage II–III Endometrial Cancer: Influence of Malignant Peritoneal Cytology. Ann Surg Oncol 28, 7591–7603 (2021). https://doi.org/10.1245/s10434-021-09900-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-021-09900-4