Abstract
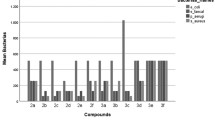
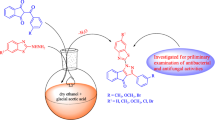
A series of indolizine derivatives have been synthesized and subjected to antibacterial screening studies. Antibacterial activity of 21 derivatives was investigated against Staphylococcus aureus, Mycobacterium smegmatis, Salmonella typhimurium and Escherichia coli; also, the sensitivity of model yeast Candida parapsilosis and some model filamentous fungi Aspergillus fumigatus, Alternaria alternata, Botrytis cinerea and Microsporum gypseum was tested. Newly synthesized indolizine derivatives have shown selective toxicity to Gram-positive bacteria S. aureus and were also considered to be able to inhibit the acidoresistant rod M. smegmatis. Derivative XXI has shown the highest inhibition effect with the bacteriostatic effect on the cells at the concentration of 25 µg mL−1. The best antifungal activity has been detected in the presence of derivative XIII. Derivative XIII did also affect the morphology of hyphal tips of B. cinerea, which led to enhanced ramification of hyphae. Finally, the antimutagenic activity of derivatives was investigated. Significant antimutagenic activity was registered in case of derivative VIII. The number of induced revertants by mutagen [2-(5-nitrofuryl)acrylic acid] was decreased almost to the level of spontaneous revertants in the lowest applied concentration (50 µg per plate).
Similar content being viewed by others
References
Brandi, A., Cicchi, S., Cordero, F. M., Frignoli, R., Goti, A., Picasso, S., & Vogel, P. (1995). Assignment of the absolute configuration of natural lentiginosine by synthesis and enzymic assays of optically pure (+) and (−)-enantiomers. Journal of Organic Chemistry, 60 6806–6812. DOI: 10.1021/jo00126a033.
Clinical Laboratory Standard Institute (2014). Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; Approved standard—Eight edition, CLSI M7-A9. Wayne, PA, USA: Clinical Laboratory Standard Institute.
Couture, A., Deniau, E., Grandclaudon, P., Lebrun, S., Léonce, S., Renard, P., & Pfeiffer, B. (2000). First synthesis and pharmacological evaluation of benzoindolizidine and benzoquinolizidine analogues of α-and β-peltatin. Bioorganic & Medicinal Chemistry, 8 2113–2125. DOI: 10.1016/s0968-0896(00)00130-9.
Darwish, E. S. (2008). Facile synthesis of heterocycles via 2-picolinium bromide and antimicrobial activities of the products. Molecules, 13 1066–1078. DOI: 10.3390/molecules13051066.
Dudová, B., Hudecová, D., Pokorný, R., Mičková, M., Palicová, M., Segl’a, P., & Melník, M. (2002). Copper complexes with bioactive ligands. Part II — Antifungal activity. Folia Microbiologica, 47 225–229. DOI: 10.1007/bf02817642.
Foster, C., Ritchie, M., Selwood, D. L., & Snowden, W. (1995). Synthesis and anti-herpes activity of a series of indolizines. Antiviral Chemistry & Chemotherapy, 6 289–297.
Gubin, J., Lucchetti, J., Mahaux, J., Nisato, D., Rosseels, G., Clinet, M., Polster, P., & Chatelain, P. (1992). A novel class of calcium-entry blockers: the 1 [[4-(aminoalkoxy)-phenyl]sulfonyl]indolizines. Journal of Medicinal Chemistry, 35 981–988. DOI: 10.1021/jm00084a002.
Gundersen, L. L., Negussie, A. H., Rise, F., & Østby, O. B. (2003). Antimycobacterial activity of 1-substituted indolizines. Archiv der Pharmazie, 336 191–195. DOI: 10.1002/ardp.200390019.
Gundersen, L. L., Charnock, C., Negussie, A. H., Rise, F., & Teklu, S. (2007). Synthesis of indolizine derivatives with selective antibacterial activity against Mycobacterium tuberculosis. European Journal of Pharmaceutical Sciences, 30 26–35. DOI: 10.1016/j.ejps.2006.09.006.
Gupta, S. P., Mathur, A. N., Nagappa, A. N., Kumar, D., & Kumaran, S. (2003). A quantitative structure-activity relationship study on a novel class of calcium-entry blockers: 1-[4-(aminoalkoxy) phenylsulphonyl]indolizines. European Journal of Medicinal Chemistry, 38 867–873. DOI: 10.1016/j.ejmech.2003.08.001.
Hazra, A., Mondal, S., Maity, A., Naskar, S., Saha, P., Paira, R., Sahu, K. B., Paira, P., Ghosh, S., Sinha, C., Samanta, A., Banerjee, S., & Mondal, N. B. (2011). Amberlite-IRA-402 (OH) ion exchange resin mediated synthesis of indolizines, pyrrolo[1,2-a]quinolines and isoquinolines: Antibacterial and antifungal evaluation of the products. European Journal of Medicinal Chemistry, 46 2132–2140. DOI: 10.1016/j.ejmech.2011.02.066.
Hema, R., Parthasarathi, V., Sarkunam, K., Nallu, M., & Linden, A. (2003). 3-(4-Chlorobenzoyl)-7-(N, N-dimethylamino)-1-phenylindolizine and 3-(2,4-dichlorobenzoyl)-7-(N,N-dimethylamino)-1-phenylindolizine. Acta Crystallographica Section C: Crystal Structure Communications, 59, o703–o705. DOI: 10.1107/s0108270103023540.
Hempel, A., Camerman, N., Mastropaolo, D., & Camerman, A. (1993). Glucosidase inhibitors: structures of deoxynojirimycin and castanospermine. Journal of Medicinal Chemistry, 36 4082–4086. DOI: 10.1021/jm00077a012.
Hudecová, D., Varečka, E., Vollek, V., & Betina, V. (1994). Growth and morphogenesis of Botrytis cinerea. Effects of exogenous calcium ions, calcium channel blockers and cyclosporin A. Folia Microbiologica, 39 269–275. DOI: 10.1007/bf02814311.
Jørgensen, A. S., Jacobsen, P., Christiansen, L. B., Bury, P. S., Kanstrup, A., Thorpe, S. M., Bain, S., Naerum, L., & Wassermann, K. (2000). Synthesis and pharmacology of a novel pyrrolo [2,1,5-cd] indolizine (NNC 45-0095), a high affinity non-steroidal agonist for the estrogen receptor. Bioorganic & Medicinal Chemistry Letters, 10 399–402. DOI: 10.1016/s0960-894x(00)00015-9.
Koul, A., Choidas, A., Treder, M., Tyagi, A. K., Drlica, K., Singh, Y., & Ullrich, A. (2000). Cloning and characterization of secretory tyrosine fosfatases of Mycobacterium tuberculosis. Journal of Bacteriology, 182 5425–5432. DOI: 10.1128/jb.182.19.5425-5432.2000.
Kubo, A., Nakai, T., Koizumi, Y., Kitahara, Y., Saito, N., Mikami, Y., Yazava, K., & Uno, J. (1996). A Synthesis of the derivatives of 1,2,3,5,10,10a-hexahydrobenz[f]indoline-6,9-dione having antifungal activity as a simple model of Saframycin A. Heterocycles, 42 195–211. DOI: 10.3987/com-94-S5-1.
Marchalin, S., Decroix, B., & Morel, J. (1993). Synthesis of indolizine-6,9-diones annelated to a thiophene ring. Acta Chemica Scandinavica, 47 287–291. DOI: 10.3891/acta.chem.scand.47-0287.
Marchalin, S., Szemes, F., Bar, N., & Decroix, B. (1999). Synthesis of enantiopure (S)-thieno[f]indolizidines. Heterocycles, 50 445–452. DOI: 10.3987/com-98-s(h)10.
Maron, D. M., & Ames, B. N. (1983). Revised methods for the Salmonella mutagenicity test. Mutation Research, 113 173–215. DOI: 10.1016/0165-1161(83)90010-9.
Mitsumori, T., Bendikov, M., Dautel, O., Wudl, F., Shioya, T., Sato, H., & Sato, Y. (2004). Synthesis and properties of highly fluorescent indolizino [3,4,5-ab] isoindoles. Journal of the American Chemical Society, 126 16793–16803. DOI: 10.1021/ja049214x.
Nasir, A. I., Gundersen, L. L., Rise, F., Antonsen, Ø., Kristensen, T., Langhelle, B., Bast, A., Custers, I., Haenen, G. R. M. M., & Wikström, H. (1998). Inhibition of lipid peroxidation mediated by indolizines. Bioorganic & Medicinal Chemistry Letters, 8 1829–1832. DOI: 10.1016/s0960-894x(98)00313-8.
Olejníková, P., Kurucová, M., Švorc, E., & Marchalín, Š. (2013). Induction of resistance in Mycobacterium smegmatis. Canadian Journal of Microbiology, 59 126–129. DOI: 10.1139/cjm-2012-0438.
Pearson, W. H., & Guo, L. (2001). Synthesis and mannosidase inhibitory activity of 3-benzyloxymethyl analogs of swainsonine. Tetrahedron Letters, 42 8267–8271. DOI: 10.1016/s0040-4039(01)01777-4.
Šafář, P., Žúžiová, J., Bobošíková, M., Marchalín, Š., Prónayová, N., Comesse, S., & Daïch, A. (2009a). Synthesis and reductive desulfurization of chiral non-racemic benzothienoindolizines. An efficient approach to a novel bioactive tylophorine alkaloid analogue and 6-phenylindolizidine. Tetrahedron: Asymmetry, 20 2137–2144. DOI: 10.1016/j.tetasy.2009.08.010.
Šafář, P., Žúžiová, J., Marchalín, Š., Tóthová, E., Prónayová, N., Švorc, E., Vrábel, V., & Daïch, A. (2009b). Highly diastereoselective approach to novel phenylindolizidinols via benzothieno analogues of tylophorine based on reductive desulfurization of benzo[b] thiophene. Tetrahedron: Asymmetry, 20 626–634. DOI: 10.1016/j.tetasy.2009.02.042.
Sonnenschein, H., Hennrich, G., Resch-Genger, U., & Schulz, B. (2000). Fluorescence and UV/Vis spectroscopic behavior of novel biindolizines. Dyes and Pigments, 46 23–27. DOI: 10.1016/s0143-7208(00)00032-2.
Teklu, S., Gundersen, L. L., Larsen, T., Malterud, K. E., & Rise, F. (2005). Indolizine 1-sulfonates as potent inhibitors of 15-lipoxygenase from soybeans. Bioorganic & Medicinal Chemistry, 13 3127–3139. DOI: 10.1016/j.bmc.2005.02.056.
Toyota, M., Komori, C., & Ihara, M. (2000). A concise formal total synthesis of mappicine and nothapodytine B via an intramolecular hetero Diels-Alder reaction. Journal of Organic Chemistry, 65 7110–7113. DOI: 10.1021/jo000816i.
Vaught, J. L., Carson, J. R., Carmosin, R. J., Blum, P. S., Persico, F. J., Hageman, W. E., Shank, R. P., & Raffa, R. B. (1990). Antinociceptive action of McN-5195 in rodents: a structurally novel (indolizine) analgesic with a nonopioid mechanism of action. Journal of Pharmacology and Experimental Therapeutics, 255 1–10.
Vemula, V. R., Vurukonda, S., & Bairi, C. K. (2011). Indolizine derivatives: recent advances and potential pharmacological activities. International Journal of Pharmaceutical Sciences Review & Research, 11 159–163.
Vlahovici, A., Andrei, M., & Druţă, I. (2002). A study of the dimethyl-3-benzoyl-5-(2′-pyridyl)-indolizine-1,2-dicarboxylate exciplexes with alcohols. Journal of Luminescence, 96 279–285. DOI: 10.1016/s0022-2313(01)00226-5.
Wavefunction (2006). Spartan’06 [computer software]. Irvine, CA, USA: Wavefunction.
Weide, T., Arve, L., Prinz, H., Waldmann, H., & Kessler, H. (2006). Substituted indolizine-1-carbonitrile derivatives as phosphatase inhibitors. Bioorganic & Medical Chemistry Letters, 16 59–63. DOI: 10.1016/j.bmcl.2005.09.051.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Olejníková, P., Birošová, L., Švorc, L. et al. Newly synthesized indolizine derivatives — antimicrobial and antimutagenic properties. Chem. Pap. 69, 983–992 (2015). https://doi.org/10.1515/chempap-2015-0093
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1515/chempap-2015-0093