Abstract
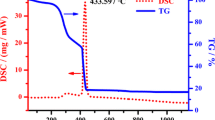
Thermal stable chloride melts were used as the reaction medium for modifying the chemical composition of complex oxides ensuring a marked improvement of their working properties. This paper discusses the original results of the direct effect of molten KCl–CoCl2 mixtures on the fine Ni4Nb2O9 powders under argon- and oxygen-containing gaseous atmospheres at 500 °C. The initial Ni4Nb2O9 powder and the reaction products were studied in detail using the differential scanning calorimetry, thermogravimetry, x-ray diffractometry, Raman and IR spectroscopies, scanning electron microscopy, energy-dispersive x-ray spectroscopy, chemical analysis, and conductometry which demonstrated clearly the formation of the thermal stable single-phase Ni–Co niobates.




Similar content being viewed by others
References
R. Wichmann and H. Mueller-Buschbaum: Zur Kristallstruktur von Ni4Nb2O9. Z. Anorg. Allg. Chem. 525, 135 (1985).
R. Wichmann and H. Mueller-Buschbaum: Eine neue Kristallstruktur des Nickel‐Oxoniobats: II‐Ni4Nb2O9. Z. Anorg. Allg. Chem. 539, 203 (1986).
A. Kan, H. Ogawa, A. Yokoi, and Y. Nakamura: Crystal structural refinement of corundum-structured A4M2O9 (A = Co and Mg, M = Nb and Ta) microwave dielectric ceramics by high-temperature X-ray powder diffraction. J. Eur. Ceram. Soc. 27, 2977 (2007).
J. Santander, E. López, A. Diez, M. Dennehy, M. Pedernera, and G. Tonetto: Ni–Nb mixed oxides: one-pot synthesis and catalytic activity for oxidative dehydrogenation of ethane. Chem. Eng. J. 255, 185 (2014).
E. Heracleous and A.A. Lemonidou: Ni–Nb–O mixed oxides as highly active and selective catalysts for ethene production via ethane oxidative dehydrogenation. Part I: characterization and catalytic performance. J. Catal. 237, 162 (2006).
H. Zhu, H. Dong, P. Laveille, Y. Saih, V. Caps, and J.-M. Basset: Metal oxides modified NiO catalysts for oxidative dehydrogenation of ethane to ethylene. Catal. Today 228, 58 (2014).
A. Qiao, V.N. Kalevaru, A. Kumar, N. Lingaiah, P.S. Prasad, and A. Martin: Effect of CO2-admixture on the catalytic performance of Ni–Nb–M–O catalysts in oxidative dehydrogenation of ethane to ethylene. DGMK Tagungsber. 3, 97 (2012).
A. Mirzaei, G.-J. Sun, J.K. Lee, C. Lee, S. Choi, and H.W. Kim: Hydrogen sensing properties and mechanism of NiO–Nb2O5 composite nanoparticle-based electrical gas sensors. Ceram. Int. 43, 5247 (2017).
A.L. Podkorytov, S.A. Shtin, A.S. Kashapova, A.A. Luppov, and N.S. Shubina: Ga- and Ti-containing Ni4Nb2O9-based solid solutions for Ni-selective electrodes. Inorg. Mater. 49, 1044 (2013).
Y.-C. Loiu: Reaction-sintering process for preparing electronic ceramics. Recent Pat. Mater. Sci. 8, 225 (2015).
H. Ehrenberg, G. Wltschek, H. Weitzel, F. Trouw, J.H. Buettner, T. Kroener, and H. Fuess: Ferrimagnetism in Ni4Nb2O9. Phys. Rev. B 52, 9595 (1995).
N.V. Tarakina, E.A. Nikulina, J. Hadermann, D.G. Kellerman, A.P. Tyutyunnik, I.F. Berger, V.G. Zubkov, and G. Van Tendeloo: Crystal structure and magnetic properties of complex oxides Mg4-xNixNb2O9, 0≤x≤4. J. Solid State Chem. 180, 3180 (2007).
V. Khokhlov, D. Modenov, V. Dokutovich, V. Kochedykov, I. Zakir’yanova, E. Vovkotrub, and I. Beketov: Lithium oxide solution in chloride melts as a medium to prepare LiCoO2 nanoparticles. MRS Commun. 4, 15 (2014).
A.R. Kamali and D.J. Fray: Preparation of lithium niobate particles via reactive molten salt synthesis method. Ceram. Int. 40, 1835 (2014).
L. Li, J. Deng, J. Chen, and X. Xing: Topochemical molten salt synthesis for functional perovskite compounds. Chem. Sci. 7, 855 (2016).
A. Burdese, M.L. Borlera, and P. Rolando: Systems between niobium oxides and the oxides of nickel and cobalt. Atti Accad. Sci. Torino: I. Classe Sci. Fis. Mat. Nat 99, 565 (1964) (in Italian).
O. Khamman, R. Yimnirun, and S. Ananta: Phase and morphology evolution of corundum-type Ni4Nb2O9 powders synthesized by solid-state reaction. Mater. Lett. 61, 2565 (2007).
M.I. Pantyukhina, A.L. Podkorytov, and V.M. Zhukovskii: Phase equilibria, charge transport, and mass transport in Sr4Nb2O9–M4Nb2O9 (M = Cd, Cu, Ni, and Zn) systems. Russ. J. Inorg. Chem 55, 103 (2010).
Y.-C. Liou, Z.-S. Tsai, K.-Z. Fung, and C.-Y. Liu: Ni4Nb2O9 ceramics prepared by the reaction-sintering process. Ceram. Int. 36, 1887 (2010).
A.L. Timofeev, A.L. Podkorytov, S.A. Shtin, V.O. Mal’tseva, A.D. Bamburov, and S.N. Marshenya: Solid-state synthesis, characterization, and properties of Ni4Nb2O9-based solid solution. Inorg. Mater. 53, 869–873 (2017).
O. Khamman, J. Jainumpone, A. Watcharapasorn, and S. Ananta: Fabrication, phase formation and microstructure of Ni4Nb2O9 ceramics fabricated by using the two-stage sintering technique. J. Korean Phys. Soc. 69, 365 (2016).
S.A. Shtin, A.L. Podkorytov, Y.S. Khlupin, S.R. Kudakaeva, E.V. Sokolova, and K.A. Khuramshina: Electrochemical properties of Ni4Nb2O9-based ceramics. Inorg. Mater. 46, 1274 (2010).
H.-J. Seifert: Über die Systeme Alkalimetallchlorid/Kobalt(II)-chlorid. Z. Anorg. Allg. Chem. 307, 137 (1961).
C. Robelin, P. Chartrand, and A.D. Pelton: Thermodynamic evaluation and optimization of the (NaCl + KCl + MgCl2 + CaCl2 + MnCl2 + FeCl2 + CoCl2 + NiCl2) system. J. Chem. Thermodyn. 36, 809 (2004).
V.Y. Shishkin and V.S. Mityaev: Purification of alkali halides by zone melting method. Izv. USSR Acad. Sci. Inorg. Mater. 18, 1917 (1982) (in Russian).
B.P. Burylev, V.L. Mironov, L. Tsemekhman, and I.T. Sryvalin: Equilibrium vapor pressures over molten salts in CoCl2–MCl systems. Izv. Vyssh. Uchebn. Zaved. Khim. Khim. Tekhnol. 18, 663 (1975) (in Russian).
L. Alexander and H.P. Klug: Basic aspects of X-ray absorption in quantitative diffraction analysis of powder mixtures. Anal. Chem. 20, 886 (1948).
E.F. Bertaut, L. Corliss, and F. Forrat: Etude niobates et tantalates de metaux de transition bivalents. J. Phys. Chem. Solids 21, 234 (1961).
N. Chaiyo, R. Muanghlua, A. Ruangphanit, W.C. Vittayakorn, and N. Vittayakorn: Synthesis, phase formation and characterization of Co4Nb2O9 powders synthesized by solid-state reaction. Adv. Mater. Res. 55–57, 873 (2008).
M. Polomska, B. Hilczer, M. Kosec, and B. Malic: Raman scattering studies of lead free (1-x)K0.5 Na0.5NbO3–xSrTiO3 relaxors. Ferroelectrics 369, 149 (2008).
J. Wang and L. Luo: The NbO6 octahedral distortion and phase structural transition of Eu3+-doped K0.5Na0.5NbO3–xLiNbO3 ferroelectric ceramics. J. Am. Ceram. Soc. 100, 1 (2017).
V.N. Tsygankov and V.V. Safonov: Electrical properties of the Nb2O5–NiO system. Inorg. Mater. 41, 1305 (2005).
A.L. Podkorytov, S.A. Shtin, V.M. Zhukovskii, and M.I. Pantyukhina: Synthesis and properties of nickel-containing niobates. Russ. J. Inorg. Chem. 44, 796 (1999).
Acknowledgment
This study was partly supported by the Russian Foundation for Basic Research (Projects No. 15-03-00368a and No. 18-03-00475a).
Author information
Authors and Affiliations
Corresponding author
Supplementary material
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1557/mrc.2019.123.
Rights and permissions
About this article
Cite this article
Khokhlov, V., Zakir’yanova, I., Dokutovich, V. et al. Modifying chemical composition of the fine Ni4Nb2O9 powders using chloride melts as reaction medium. MRS Communications 9, 1300–1305 (2019). https://doi.org/10.1557/mrc.2019.123
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/mrc.2019.123