Abstract
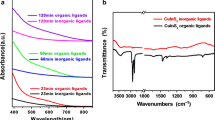
To investigate the manipulation of electromagnetic properties of two-dimensional materials, this effort characterizes charge transfer behavior of colloidal COF-5 (covalent organic framework) in the presence of various metal ions. A series of metal chloride compounds was introduced to COF-5 in solution and solid film phases and the interaction of the material with electromagnetic radiation was monitored across the visible region using electronic absorption spectroscopy. Notable changes were observed, quantified, and discussed for copper (II) chloride (CuCI2), chromium (III) chloride (CrCI3), and iron (III) chloride (FeCI3) with COF-5. Ligand-to-metal and metal-to-ligand charge transfer are explored as a possible mechanism for the observed electronic behaviors.




Similar content being viewed by others
References
L. Wang, Z. Wei, M. Mao, H. Wang, Y. Li, and J. Ma: Metal oxide/graphene composite anode materials for sodium-ion batteries. Energy Storage Mater. 16, 434–454 (2019).
H. Tang, Q. Hu, M. Zheng, Y. Chi, X. Qin, H. Pang, and Q. Xu: MXene-2D layered electrode materials for energy storage. Prog. Nat. Sci. 28, 133–147 (2018).
Y.-H. Wang, K.-J. Huang, and X. Wu: Recent advances in transition-metal dichalcogenides based electrochemical biosensors: a review. Biosens. Bioelectron. 97, 305–316 (2017).
M.M. Shulaker, G. Hills, N. Patil, H. Wei, H.-Y. Chen, H.-S.P. Wong, and S. Mitra: Carbon nanotube computer. Nature 501, 526–530 (2013).
S. Sankaran, K. Deshmukh, M.B. Ahamed, and S.K. Pasha: Recent advances in electromagnetic interference shielding properties of metal and carbon filler reinforced flexible polymer composites: a review. Composites Part A 114, 49–71 (2018).
Y. Song, L. He, X. Zhang, F. Liu, N. Tian, Y. Tang, and J. Kong: Highly efficient electromagnetic wave absorbing metal-free and carbon-rich ceramics derived from hyperbranched polycarbosilazanes. J. Phys. Chem. C 121, 24774–24785 (2017).
A.P. Cote, A.I. Benin, N.I. Ockwig, M. O’Keeffe, A.J. Matzger, and O.M. Yaghi: Porous, crystalline, covalent organic frameworks. Science 310, 1166–1170 (2005).
S. Wan, J. Guo, J. Kim, H. Ihee, and D. Jiang: A photoconductive covalent organic framework: self-condensed arene cubes composed of eclipsed 2D polypropylene sheets for photocurrent generation. Angew. Chem. Int. Ed. 48, 5439–5442 (2009).
R.-L. Wang, D.-P. Li, L.-J. Wang, X. Zhang, Z.-Y. Zhou, J.-L. Mu, and Z.-M.S. Su: The preparation of new covalent organic framework embedded with silver nanoparticles and its applications in degradation of organic pollutants from waste water. Dalton Trans. 48, 1051–1059 (2019).
X. Ding, J. Guo, X. Feng, Y. Honsho, J. Guo, S. Seki, P. Maitarad, A. Saeki, S. Nagase, and D. Jiang: Synthesis of metallophthalocyanine covalent organic frameworks that exhibit high carrier mobility and photoconductivity. Angew. Chem. Int. Ed. 50, 1289–1293 (2010).
E.L. Spitler and W.R. Dichtel: Lewis acid-catalysed formation of two-dimensional phthalocyanine covalent organic frameworks. Nat. Chem. 2, 672–677 (2010).
H. Yang, S. Zhang, L. Han, Z. Zhang, Z. Xue, J. Gao, Y. Li, C. Huang, Y. Yi, H. Liu, and Y. Li: High conductive two-dimensional covalent organic framework for lithium storage with large capacity. ACS Appl. Mater. Interfaces 8, 5366–5375 (2016).
L. Ascherl, E.W. Evans, M. Hennemann, D. Di Nuzzo, A.G. Hufnagel, M. Beetz, R.H. Friend, T. Clark, T. Bein, and F. Auras: Solvatochromic covalent organic frameworks. Nat. Commun. 9, 3802 (2018).
S. Dalapati, E. Jin, M. Addicoat, T. Heine, and D. Jiang: Highly emissive covalent organic frameworks. J. Am. Chem. Soc. 138, 5797–5800 (2016).
M.S. Dresselhaus and G. Dresselhaus: Intercalation compounds of graphite. Adv. Phys. 51, 1–186 (2002).
J. Xu, Y. Dou, Z. Wei, J. Ma, Y. Deng, Y. Li, H. Liu, and S. Dou: Recent progress in graphite intercalation compounds for rechargeable metal (Li, Na, K, Al)-ion batteries. Adv. Sci. 4, 1700146, 1–14 (2017).
B.J. Smith, L.R. Parent, A.C. Overholts, P.A. Beaucage, R.P. Bisbey, A.D. Chavez, N. Hwang, C. Park, A.M. Evans, N.C. Gianneschi, and W.R. Dichtel: Colloidal covalent organic frameworks. ACS Cent. Sci. 3, 58–65 (2017).
B. Lukose, A. Kuc, J. Frenzel, and T. Heine: On the reticular construction concept of covalent organic frameworks. Beilstein J. Nanotechnol. 1, 60–70 (2010).
Y. Zhou, Z. Wang, P. Yang, X. Zu, and F. Gao: Electronic and optical properties of two-dimensional covalent organic frameworks. J. Mater. Chem. 22, 16964–16970 (2012).
Z. Xu: Mechanics of metal-catecholate complexes: the roles of coordination state and metal types. Sci. Rep. 3, 2914 (2013).
D. Shriver, and P. Atkins: Inorganic Chemistry. 3rd ed. (W. H. Freeman and Company, New York, NY, 1999).
D.M. Manuta, and A.J. Lees: Solvatochromism of the metal to ligand charge-transfer transitions of zerovalent tungsten carbonyl complexes. Inorg. Chem. 25, 3212–3218 (1986).
B. Carlotti, R. Flamini, I. Kikas, U. Mazzucato, and A. Spalletti: Intramolecular charge transfer, solvatochromism and hyperpolarizability of compounds bearing ethenylene or ethynylene bridges. Chem. Phys. 407, 9–19 (2012).
OpenStax College: Chemistry (OpenStax, Houston, TX, 2015).
J.K. Utterback, M.B. Wilker, D.W. Mulder, P.W. King, J.D. Eaves, and G. Dukovic: Quantum efficiency of charge transfer competing against nonexponential processes: the case of electron transfer from CdS nanorods to hydrogenase. J. Phys. Chem. 123, 886–896 (2019).
Acknowledgments
The authors gratefully acknowledge funding for this effort through the Naval Surface Warfare Center–Dahlgren Division (NSWCDD) In-House Laboratory Independent Research (ILIR) program.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Supplementary material
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1557/mrc.2020.7.
Rights and permissions
About this article
Cite this article
Owen, W.S., Bible, M.S., Dohmeier, E.F. et al. Electronic charge transfer properties of COF-5 solutions and films with intercalated metal ions. MRS Communications 10, 91–97 (2020). https://doi.org/10.1557/mrc.2020.7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/mrc.2020.7