Abstract
Background
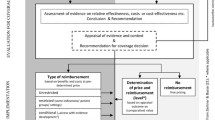
Value-based pricing (VBP), whereby prices are set according to the perceived benefits offered to the consumer at a time when costs and benefits are characterized by considerable uncertainty and are then reviewed ex post, is a much discussed topic in pharmaceutical reimbursement. It is usually combined with coverage with evidence development (CED), a tool in which manufacturers are granted temporary reimbursement but are required to collect and submit additional health economic data at review. Many countries, including the UK, are signalling shifts in this direction. Several countries, including Sweden, have already adopted this approach and offer good insight into the benefits and pitfalls in actual practice.
Objective
To describe VBP reimbursement decision making using CED in actual practice in Sweden.
Methods
Decision making by The Dental and Pharmaceutical Benefits Agency (TLV) in Sweden was reviewed using a case study of continuous intraduodenal infusion of levodopa/carbidopa (Duodopa®) in the treatment of advanced Parkinson’s disease (PD) with severe motor fluctuations.
Results
The manufacturer of Duodopa® applied for reimbursement in late 2003. While the proper economic data were not included in the submission, TLV granted reimbursement until early 2005 to provide time for the manufacturer to submit a formal economic evaluation. The re-submission with economic data was considered inadequate to judge cost effectiveness, so TLV granted an additional extension of reimbursement until August 2007, at which time conclusive data were expected. The manufacturer initiated a 3-year, prospective health economic study and a formal economic model. Data from a pre-planned interim analysis of the data were loaded into the model and the cost-effectiveness ratio was the basis of the next re-submission. TLV concluded that the data were suitable for making a definite decision and that the drug was not cost effective, deciding to discontinue reimbursement for any new patients (current patients were unaffected). The manufacturer continued to collect data and to improve the economic model and re-submitted in 2008. New data and the improved model resulted in reduced uncertainty and a lower cost-effectiveness ratio in the range of Swedish kronor (SEK)430 000 per QALY gained in the base-case analysis, ranging up to SEK900 000 in the most conservative sensitivity analysis, resulting in reimbursement being granted.
Discussion
The case of Duodopa® provides excellent insight into VBP reimbursement decision making in combination with CED and ex post review in actual practice. Publicly available decisions document the rigorous, time-consuming process (four iterations were required before a final decision could be reached). The data generated as part of the risk-sharing agreement proved correct the initial decision to grant limited coverage despite lack of economic data. Access was provided to 100 patients while evidence was generated.
Conclusions
Economic appraisal differs from clinical assessment, and decision makers benefit from analysis of naturalistic, actual practice data. Despite reviewing the initial trial-based, ‘piggy-back’ economic analysis, TLV was uncertain of the cost effectiveness in actual practice and deferred a final decision until observational data from the DAPHNE study became available. Second, acceptance of economic modelling and use of temporary reimbursement conditional on additional evidence development provide a mechanism for risk sharing between TLV and manufacturers, which enabled patient access to a drug with proven clinical benefit while necessary evidence to support claims of cost effectiveness could be generated.


Similar content being viewed by others
Notes
TLV neither directly sets nor negotiates price.
A small number of patients were recruited in neighbouring Norway.
Some modelling was required to overcome gaps in data coverage for many of the cells (health states) required by the model.
References
Claxton K. OFT, VBP: QED? Health Econ 2007; 16(6): 545–58
Organisation for Economic Co-operation and Development. OECD health policy studies: pharmaceutical pricing policies in a global market. Paris: OECD, 2008
Office of Fair Trading. The pharmaceutical price regulation scheme: an OFT market study. London: OFT, 2007 Feb
Hutton J, Trueman P, Henshall C. Coverage with evidence development: an examination of conceptual and policy issues. Int J Technol Assess Health Care 2007; 23: 425–32
Dental and Pharmaceutical Benefits Agency (TLV). Review of drugs that reduce blood pressure: summary [in Swedish; online]. Available from URL: http://tlv.se/Upload/Genomgangen/sammanfattning-blodtryck.pdf [Accessed 2010 Aug 10]
Schrag A, Ben-Shlomo Y, Quinn N. How common are complications of Parkinson’s disease? Neurology 2002; 249: 419–23
Waters CH. Treatment of advanced stage patients with Parkinson’s disease. Parkinsonism Relat Disord 2002 Oct; 9(1): 15–21
Katzenschlager R, Lees AJ. Treatment of Parkinson’s disease: levodopa as the first choice. J Neurol 2002; 249Suppl. 2: 20–4
Antonini A, Odin P. Pros and cons of apomorphine and L-dopa continuous infusion in advanced Parkinson’s disease. Parkinsonism Relat Disord 2009 Dec; 15Suppl. 4: S97–100
Clarke CE, Worth P, Grosset D, et al. Systematic review of apomorphine infusion, levodopa infusion and deep brain stimulation in advanced Parkinson’s disease. Parkinsonism Relat Disord 2009 Dec; 15(10): 728–41
Dental and Pharmaceutical Benefits Agency (TLV). Läkemedelsförmånsnämnden decision #1680/2004 [online]. Available from URL: http://www.tlv.se/Upload/Beslut_2005/BES_050131_duodopa.pdf [Accessed 2009 Feb 13]
Nyholm D, Nilsson R, Dizdar N, et al. Duodenal levodopa infusion monotherapy vs oral polypharmacy in advanced Parkinson Disease. Neurology 2005; 64: 216–23
Dental and Pharmaceutical Benefits Agency (TLV). Läkemedelsförmånsnämnden decision #1443/2003 [online]. Available from URL: http://www.tlv.se/Upload/Beslut/BES_040123_Duodopa.pdf [Accessed 2009 Feb 13]
Kristiansen IS, Bingefors K, Nyholm D, et al. Short-term cost and health consequences of duodenal levodopa infusion in advanced Parkinson’s Disease in Sweden: an exploratory study. Appl Health Econ Health Policy 2009; 7(3): 167–80
Pålhagen SE, Dizdar N, Hauge TB, et al. Long-term study on clinical benefits and quality-of-life of intraduodenal levodopa in routine care for a cohort of treatment-naïve patients with advanced parkinson’s disease [poster]. American Academy of Neurology Meeting; 2010 Apr 11–16; Toronto (ON)
Hagell P, Nordling S, Reimer J, et al. Resource use and costs in a swedish cohort of patients with Parkinson’s Disease. Mov Disord 2002; 17: 1213–20
Dental and Pharmaceutical Benefits Agency (TLV). Läkemedelsförmånsnämnden decision #984/2007 [online]. Available from URL: http://www.tlv.se/Upload/Beslut_2007/BES_070904_duodopa.pdf [Accessed 2009 Feb 13]
Antonini A, Ioannis U, Cannesi M, et al. Duodenal levodopa infusion for advanced Parkinson’s: 12-month treatment outcome. Move Disord 2007; 22: 1145–9
Dental and Pharmaceutical Benefits Agency (TLV). Läkemedelsförmånsnämnden decision #395/2008 [online]. Available from URL: http://www.tlv.se/Upload/Beslut_2008/bes080625-duodopa.pdf [Accessed 2009 Feb 13]
Dental and Pharmaceutical Benefits Agency (TLV) [online]. Available from URL: http://www.tlv.se/lakemedel/ansok-om-pris-eller-subvention/radgivning-for-foretag/ [Accessed 2010Aug10]
Acknowledgements
This work was funded by, and two of the authors (YZ and BG) are employees of, Solvay Pharmaceuticals (Solvay Pharmaceuticals is now Abbott). The other two authors are employees of The Swedish Institute for Health Economics, a not-for-profit research institute, which has conducted projects commissioned by Solvay Pharmaceuticals. This work has benefited greatly from the comments of two anonymous referees. Any remaining errors and omissions belong to the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Willis, M., Persson, U., Zoellner, Y. et al. Reducing uncertainty in value-based pricing using evidence development agreements. Appl Health Econ Health Policy 8, 377–386 (2010). https://doi.org/10.2165/11531160-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11531160-000000000-00000