Abstract
Purpose
Light microscopic manual count is the current gold standard for parasite quantification. The ability to determine parasite density in whole blood is crucial to understanding disease pathogenesis and finding a suitable automated method of Babesia rossi parasite quantification would facilitate higher throughput and provide results that are more objective. This study investigated both peripheral capillary and central venous whole blood to estimate the correlations between light microscopy, flow cytometry and quantitative real-time polymerase chain reaction (qPCR).
Methods
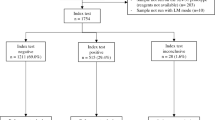
Peripheral capillary and central venous blood were sampled from 40 naturally B. rossi-infected dogs and 10 healthy control dogs. Samples were analysed by reverse line blot hybridization assay to confirm a mono-B. rossi infection. Capillary blood parasite density was detected using light microscopic manual counting and venous blood parasitaemia detected by manual counts, flow cytometry and qPCR.
Results
A significant correlation was found between the venous manual counts and flow cytometry (rs = 0.465; P < 0.001), as well as qPCR (rs = − 0.500; P < 0.001). A significant correlation was also observed between the capillary manual counts compared to venous manual counts (rs = 0.793; P < 0.001), flow cytometry (rs = 0.399; P = 0.004), and qPCR (rs = − 0.526; P < 0.001).
Conclusions
The study results suggest that qPCR is of value as an alternative to the gold standard manual count for detecting B. rossi parasitaemia in canine whole blood and that flow cytometry may be useful with further refinement of issues such as background fluorescence and the influence of reticulocytes.



Similar content being viewed by others
Data Availability
The datasets generated during and/or analysed during the current study care are available in the University of Pretoria repository, http://hdl.handle.net/2263/66680.
References
Allred DR, Al-Khedery B (2004) Antigenic variation and cytoadhesion in Babesia bovis and Plasmodium falciparum: different logics achieve the same goal. Mol Biochem Parasitol 134:27–35
Bicalho KA, Ribeiro MF, Martins-Filho OA (2004) Molecular fluorescent approach to assessing intraerythrocytic hemoprotozoan Babesia canis infection in dogs. Vet Parasitol 125:221–235
Birkenheuer AJ, Levy MG, Breitschwerdt EB (2003) Development and evaluation of a seminested PCR for detection and differentiation of Babesia gibsoni (Asian Genotype) and B. canis DNA in canine blood samples. J Clin Microbiol 41:4172–4177
Bohm M, Leisewitz AL, Thompson PN, Schoeman JP (2006) Capillary and venous Babesia canis rossi parasitaemias and their association with outcome of infection and circulatory compromise. Vet Parasitol 141:18–29
Borggraefe I, Yuan J, Telford SR et al (2006) Babesia microti primarily invades mature erythrocytes in mice. Infect Immun 74:3204–3212
Callow LL, Pepper PM (1974) Measurement of and correlations between fever, changes in the packed cell volume and parasitaemia in the evaluation of the susceptibility of cattle to infection with Babesia argentina. Aust Vet J 50(1):1–5
Campo JJ, Aponte JJ, Nhabomba AJ et al (2011) Feasibility of flow cytometry for measurements of Plasmodium falciparum parasite burden in studies in areas of malaria endemicity by use of bidimensional assessment of YOYO-1 and autofluorescence. J Clin Microbiol 49(3):968–974
Clark I, Jacobson L (1998) Do babesiosis and malaria share a common disease process? Ann Trop Med Parasitol 92(4):483–488
Costa LM Jr, Zahler-Rinder M, Ribeiro MF et al (2012) Use of a real time PCR for detecting subspecies of Babesia canis. Vet Parasitol 188:160–163
Costa-Junior LM, Rabelo EM, Martins Filho OA, Ribeiro MF (2006) Comparison of different direct diagnostic methods to identify Babesia bovis and Babesia bigemina in animals vaccinated with live attenuated parasites. Vet Parasitol 139(1–3):231–236
Cunnington AJ, Walther M, Riley EM (2013) Piecing together the puzzle of severe malaria. Sci Transl Med 5(211):211ps218
Duarte SC, Linhares GF, Romanowsky TN, da Silveira Neto OJ, Borges LM (2008) Assessment of primers designed for the subspecies-specific discrimination among Babesia canis canis, Babesia canis vogeli and Babesia canis rossi by PCR assay. Vet Parasitol 152:16–20
Gohil S, Kats LM, Sturm A, Cooke BM (2010) Recent insights into alteration of red blood cells by Babesia bovis: moovin’ forward. Trends Parasitol 26:591–599
Grimberg BT (2011) Methodology and application of flow cytometry for investigation of human malaria parasites. J Immunol Methods 367(1–2):1–16
Hutchings CL, Li A, Fernandez KM et al (2007) New insights into the altered adhesive and mechanical properties of red blood cells parasitized by Babesia bovis. Mol Microbiol 65:1092–1105
Hwang SY, Kim SH, Lee GY et al (2011) A novel real-time PCR assay for the detection of Plasmodium falciparum and Plasmodium vivax malaria in low parasitized individuals. Acta Trop 120(1–2):40–45
Izumiyama S, Omura M, Takasaki T, Ohmae H, Asahi H (2009) Plasmodium falciparum: development and validation of a measure of intraerythrocytic growth using SYBR Green I in a flow cytometer. Exp Parasitol 121(2):144–150
Jiménez-Díaz MB, Rullas J, Mulet T et al (2005) Improvement of detection specificity of Plasmodium-infected murine erythrocytes by flow cytometry using autofluorescence and YOYO-1. Cytometry Part A 67(1):27–36
Krause PJ, Daily J, Telford SR et al (2007) Shared features in the pathobiology of babesiosis and malaria. Trends Parasitol 23(12):605–610
Lyke KE, Diallo DA, Dicko A et al (2003) Association of intraleukocytic Plasmodium falciparum malaria pigment with disease severity, clinical manifestations, and prognosis in severe malaria. Am J Trop Med Hyg 69(3):253–259
Malleret B, Claser C, Ong AS et al (2011) A rapid and robust tri-color flow cytometry assay for monitoring malaria parasite development. Sci Rep 1:118
Matjila PT, Carcy B, Leisewitz AL et al (2009) Preliminary evaluation of the BrEMA1 gene as a tool for associating Babesia rossi genotypes and clinical manifestation of canine babesiosis. J Clin Microbiol 47:3586–3592
Matjila PT, Leisewitz AL, Jongejan F, Penzhorn BL (2008) Molecular detection of tick-borne protozoal and ehrlichial infections in domestic dogs in South Africa. Vet Parasitol 155:152–157
Mendis K, Carter R (1995) Clinical disease and pathogenesis in malaria. Parasitol Today 11(5):PTI1–PTI16
Miller LH, Baruch DI, Marsh K, Doumbo OK (2002) The pathogenic basis of malaria. Nature 415(6872):673–679
Mosqueda J, Olvera-Ramirez A, Aguilar-Tipacamu G, Canto GJ (2012) Current advances in detection and treatment of babesiosis. Curr Med Chem 19:1504–1518
O’Connor RM et al (1999) Cytoadherence of Babesia bovis-infected erythrocytes to bovine brain capillary endothelial cells provides an in vitro model for sequestration. Infect Immun 67(8): 3921–3928
Osoga J, Waitumbi J, Guyah B et al (2017) Comparative evaluation of fluorescent in situ hybridization and Giemsa microscopy with quantitative real-time PCR technique in detecting malaria parasites in a holoendemic region of Kenya. Malar J 16(1):297
O’meara WP, McKenzie FE, Magill AJ (2005) Sources of variability in determining malaria parasite density by microscopy. Am J Trop Med Hyg 73(3):593–598
Payne D (1988) Use and limitations of light microscopy for diagnosing malaria at the primary health care level. Bull World Health Organ 66(5):621
Philipp S, Oberg HH, Janssen O, Leippe M, Gelhaus C (2012) Isolation of erythrocytes infected with viable early stages of Plasmodium falciparum by flow cytometry. Cytometry Part A 81(12):1048–1054
Rautenbach Y, Goddard A, Thompson PN, Mellanby RJ, Leisewitz AL (2017) A flow cytometric assessment of the lymphocyte immunophenotypes in dogs naturally infected with Babesia rossi. Vet Parasitol 241:26–34
Rebelo M, Shapiro HM, Amaral T, Melo-Cristino J, Hänscheid T (2012) Haemozoin detection in infected erythrocytes for Plasmodium falciparum malaria diagnosis—prospects and limitations. Acta Trop 123(1):58–61
Reyers F, Leisewitz A, Lobetti R, Milner R, Jacobson L (1998) Canine babesiosis in South Africa: more than one disease. Does this serve as a model for falciparum malaria? Ann Trop Med Parasitol 92(4):503–511
Roobsoong W, Maher SP, Rachaphaew N et al (2014) A rapid sensitive, flow cytometry-based method for the detection of Plasmodium vivax-infected blood cells. Malar J 13(1):55
Rossouw I, Maritz-Olivier C, Niemand J et al (2015) Morphological and molecular descriptors of the developmental cycle of Babesia divergens parasites in human erythrocytes. PLoS Negl Trop Dis 9:e0003711
Schneider P, Wolters L, Schoone G et al (2005) Real-time nucleic acid sequence-based amplification is more convenient than real-time PCR for quantification of Plasmodium falciparum. J Clin Microbiol 43(1):402–405
Shapiro HM, Apte SH, Chojnowski GM et al (2013) Cytometry in malaria—a practical replacement for microscopy? Curr Protoc Cytom 65:11–20
Sherman IW, Eda S, Winograd E (2003) Cytoadherence and sequestration in Plasmodium falciparum: defining the ties that bind. Microbes Infect 5(10):897–909
Sori G, Zewdie O, Tadele G, Samuel A (2018) External quality assessment of malaria microscopy diagnosis in selected health facilities in Western Oromia, Ethiopia. Malar J 17(1):233
Tavares R, Staggemeier R, Borges A et al (2011) Molecular techniques for the study and diagnosis of parasite infection. J Venom Anim Toxins Incl Trop Dis 17(3):239–248
Theron M, Hesketh RL, Subramanian S, Rayner JC (2010) An adaptable two-color flow cytometric assay to quantitate the invasion of erythrocytes by Plasmodium falciparum parasites. Cytom Part A 77(11):1067–1074
Troskie M, De Villiers L, Leisewitz AL, Oosthuizen MC, Quan M (2019) Development and analytical validation of a multiplex, real-time PCR assay for Babesia rossi and Babesia vogeli. Ticks Tick Borne Dis 10(2):421–432
Wang C, Ahluwalia SK, Li Y et al (2010) Frequency and therapy monitoring of canine Babesia spp. infection by high-resolution melting curve quantitative FRET-PCR. Vet Parasitol 168:11–18
Wongchotigul V et al (2004) The use of flow cytometry as a diagnostic test for malaria parasites. South-east Asian J Trop Med Public Health 35(3):552–559
Yamasaki M, Otsuka Y, Yamato O, Tajima M, Maede Y (2000) The cause of the predilection of Babesia gibsoni for reticulocytes. J Vet Med Sci 62:737–741
Acknowledgements
This work was supported by the National Research Foundation of South Africa under a grant held by ALL (CPRR13080726333), as well as by the Department of Companion Animal Studies, Faculty of Veterinary Science, Onderstepoort, South Africa. We thank Dr. Jeanne Rudman from Mamelodi Animal Health Clinic and Sr. Marizelle de Clercq from the Onderstepoort Animal Blood Bank for assistance in sample collections. We also thank Prof. Marinda Oosthuizen, Prof. Geoffrey Fosgate and Mr. Pieter de Villiers for their contributions. A special thanks to Doornpoort Animal Clinic for allowing us to sample at the clinic.
Funding
This work was supported by the National Research Foundation of South Africa under a grant held by ALL (CPRR13080726333), as well as by the Department of Companion Animal Studies, Faculty of Veterinary Science, Onderstepoort, South Africa.
Author information
Authors and Affiliations
Contributions
LDV wrote the manuscript, with input from ALL, as part of fulfilment of the requirements for obtaining the degree M.Sc. Veterinary Science. LDV assisted in sample collection and performed the light microscopic and flow cytometric laboratory work. ALL supervised the study and assisted with sample collections. MQ co-supervised the study and developed the qPCR protocol along with MT, who performed all the RLB and qPCR laboratory work. JCJ performed the statistical analysis for the study, with input from LDV.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with ethical required in terms of the University of Pretoria’s Code of ethics for researchers and the Policy guidelines for responsible research and ethical clearance was granted by the Animal Ethics Committee of the Faculty of Veterinary Science, Onderstepoort (Reference: V060-16).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Villiers, L., Quan, M., Troskie, M. et al. A Comparison Between Manual Count, Flow Cytometry and Quantitative Real-Time Polymerase Chain Reaction as a Means of Determining Babesia rossi Parasitaemia in Naturally Infected Dogs. Acta Parasit. 65, 128–135 (2020). https://doi.org/10.2478/s11686-019-00134-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11686-019-00134-9