Abstract
Introduction
Protozoan parasites of the Order Trypanosomatida infect a wide range of multicellular plants and animals, causing devastating and potentially fatal diseases. Trypanosomes are the most relevant members of the order in sub-Saharan Africa because of mortalities and morbidities caused to humans and livestock.
Purpose
There are growing concerns that trypanosomes are expanding their reservoirs among wild animals, which habours the parasites, withstand the infection, and from which tsetse flies transmit the parasites back to humans and livestock. This study was designed to investigate the potentials of the African hedgehog serving as reservoir for African animal trypanosomes.
Methods
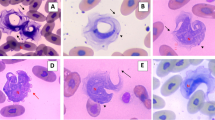
Five adult hedgehogs alongside five laboratory mice were intraperitoneally inoculated with 106 and 104 of Trypanosoma congolense cells, respectively, and monitored for parasitemia and survival. Serum from twenty hedgehogs was subjected to trypanocidal activity-guided fractionation by successive ion-exchange and gel-filtration chromatographies, followed by characterization with Sodium Dodecyl Sulphate Polyacrylamide Gel Electrophoresis (SDS-PAGE).
Results
Hedgehogs were resistant to the infection as no parasite was detected and none died even after 60 days, while all the mice died within 12 days. Both the serum and plasma prepared from hedgehogs demonstrated trypanocidal activity- rapidly killed trypanosomes even when diluted 1000 times. The trypanolytic factor was identified to be proteinaceous with an estimated molecular weight of 115-kDa.
Conclusion
For the first time, it is here demonstrated that hedgehog blood has significant trypanolytic activity against T. congolense. The potential application of the hedgehog protein for the breeding of trypanosomosis-resistant livestock in tsetse fly belt is discussed.





Similar content being viewed by others
References
Lukes J, Butenko A, Hashimi H, Maslov DA, Votypka J, Yurchenko V (2018) Trypanosomatids are much more than just trypanosomes: clues from the expanded family tree. Trends Parasitol 34:466–480. https://doi.org/10.1016/j.pt.2018.03.002
Schaub GA (1994) Pathogenicity of trypanosomatids on insects. Parasitol Today 10:463–468. https://doi.org/10.1016/0169-4758(94)90155-4
Maslov DA, Votypka J, Yurchenko V, Lukes J (2013) Diversity and phylogeny of insect trypanosomatids: all that is hidden shall be revealed. Trends Parasitol 29:43–52. https://doi.org/10.1016/j.pt.2012.11.001
Stuart K, Brun R, Croft S, Fairlamb A, Gurtler RE, McKerrow J et al (2008) Kinetoplastids: related protozoan pathogens, different diseases. J Clin Investig 118:1301–1310. https://doi.org/10.1172/JCI33945
Van Den Berghe L, Zaghi AJ (1963) Wild pigs as hosts of glossina vanhoofi henrard and trypanosoma suis ochmann in the central african forest. Nature 197:1126–1127. https://www.nature.com/articles/1971126a0
McInnes LM, Gillett A, Ryan UM, Austen J, Campbell RS, Hanger J et al (2009) Trypanosoma irwini. n sp (Sarcomastigophora: Trypanosomatidae) from the koala (Phascolarctos cinereus). Parasitology 136:875–885. https://doi.org/10.1017/S0031182009006313
Hagos A, Degefa G, Yacob H, Fikru R, Alemu T, Feseha G et al (2010) Seroepidemiological survey of trypanozoon infection in horses in the suspected dourine-infected Bale highlands of the Oromia region. Ethiopia Revue Sci Tech 29:649–654. https://doi.org/10.20506/rst.29.3.2005
Valkiunas G, Iezhova TA, Carlson JS, Sehgal RN (2011) Two new trypanosoma species from African birds, with notes on the taxonomy of avian trypanosomes. J Parasitol 97:924–930. https://doi.org/10.1645/GE-2796.1
Lemos M, Fermino BR, Simas-Rodrigues C, Hoffmann L, Silva R, Camargo EP et al (2015) Phylogenetic and morphological characterization of trypanosomes from Brazilian armoured catfishes and leeches reveal high species diversity, mixed infections and a new fish trypanosome species. Parasites Vectors 8:573. https://doi.org/10.1186/s13071-015-1193-7
Kamidi CM, Saarman NP, Dion K, Mireji PO, Ouma C, Murilla G et al (2017) Multiple evolutionary origins of Trypanosoma evansi in Kenya. PLoS Negl Trop Dis 11:e0005895. https://doi.org/10.1371/journal.pntd.0005895
Odeniran PO, Ademola IO, Macleod ET, Welburn SC (2018) Bovine and small ruminant African animal trypanosomiasis in Nigeria—a review. Vet Parasitol Regional Stud Rep 13:5–13. https://doi.org/10.1016/j.vprsr.2018.03.001
Ebhodaghe F, Ohiolei JA, Isaac C (2018) A systematic review and meta-analysis of small ruminant and porcine trypanosomiasis prevalence in sub-Saharan Africa (1986 to 2018). Acta Trop 188:118–131. https://doi.org/10.1016/j.actatropica.2018.08.034
Yasine A, Ashenafi H, Geldhof P, Bekana M, Tola A, Van Brantegem L et al (2019) Reduction of Trypanosoma equiperdum from equine semen by single layer centrifugation. Exp Parasitol 200:79–83. https://doi.org/10.1016/j.exppara.2019.04.002
Odeniran PO, Macleod ET, Ademola IO, Welburn SC (2019) Molecular identification of bovine trypanosomes in relation to cattle sources in southwest Nigeria. Parasitol Int 68:1–8. https://doi.org/10.1016/j.parint.2018.09.005
Smith AB, Esko JD, Hajduk SL (1995) Killing of trypanosomes by the human haptoglobin-related protein. Science 268:284–286. https://doi.org/10.1126/science.7716520
Pays E, Vanhollebeke B, Vanhamme L, Paturiaux-Hanocq F, Nolan DP, Perez-Morga D (2006) The trypanolytic factor of human serum. Nat Rev Microbiol 4:477–486. https://doi.org/10.1038/nrmicro1428
Shiflett AM, Bishop JR, Pahwa A, Hajduk SL (2005) Human high density lipoproteins are platforms for the assembly of multi-component innate immune complexes. J Biol Chem 280:32578–32585. https://doi.org/10.1074/jbc.M503510200
Molina-Portela MP, Samanovic M, Raper J (2008) Distinct roles of apolipoprotein components within the trypanosome lytic factor complex revealed in a novel transgenic mouse model. J Exp Med 205:1721–1728. https://doi.org/10.1084/jem.20071463
Rifkin MR (1978) Identification of the trypanocidal factor in normal human serum: high density lipoprotein. Proc Natl Acad Sci USA 75:3450–3454. https://doi.org/10.1073/pnas.75.7.3450
Vanhamme L, Paturiaux-Hanocq F, Poelvoorde P, Nolan DP, Lins L, Van Den Abbeele J et al (2003) Apolipoprotein L-I is the trypanosome lytic factor of human serum. Nature 422:83–87. https://doi.org/10.1038/nature01461
Vanhollebeke B, De Muylder G, Nielsen MJ, Pays A, Tebabi P, Dieu M et al (2008) A haptoglobin-hemoglobin receptor conveys innate immunity to Trypanosoma brucei in humans. Science 320:677–681. https://doi.org/10.1126/science.1156296
Vanhollebeke B, Pays E (2010) The trypanolytic factor of human serum: many ways to enter the parasite, a single way to kill. Mol Microbiol 76:806–814. https://doi.org/10.1111/j.1365-2958.2010.07156.x
Gibson W (2007) Resolution of the species problem in African trypanosomes. Int J Parasitol 37:829–838. https://doi.org/10.1016/j.ijpara.2007.03.002
Goodhead I, Capewell P, Bailey JW, Beament T, Chance M, Kay S, et al (2013) Whole-genome sequencing of Trypanosoma brucei reveals introgression between subspecies that is associated with virulence. mBio. 4:e00197–13. https://doi.org/10.1128/mBio.00197-13
Weir W, Capewell P, Foth B, Clucas C, Pountain A, Steketee P et al (2016) Population genomics reveals the origin and asexual evolution of human infective trypanosomes. eLife 5:e11473. https://doi.org/10.7554/eLife.11473
Lugli EB, Pouliot M, Portela Mdel P, Loomis MR, Raper J (2004) Characterization of primate trypanosome lytic factors. Mol Biochem Parasitol 138:9–20. https://doi.org/10.1016/j.molbiopara.2004.07.004
Cooper A, Capewell P, Clucas C, Veitch N, Weir W, Thomson R et al (2016) A Primate APOL1 variant That Kills Trypanosoma brucei gambiense. PLoS Negl Trop Dis 10:e0004903. https://doi.org/10.1371/journal.pntd.0004903
Thomson R, Molina-Portela P, Mott H, Carrington M, Raper J (2009) Hydrodynamic gene delivery of baboon trypanosome lytic factor eliminates both animal and human-infective African trypanosomes. Proc Natl Acad Sci USA 106:19509–19514. https://doi.org/10.1073/pnas.0905669106
Thomson R, Genovese G, Canon C, Kovacsics D, Higgins MK, Carrington M et al (2014) Evolution of the primate trypanolytic factor APOL1. Proc Natl Acad Sci USA 111:E2130–E2139. https://doi.org/10.1073/pnas.1400699111
Raper J, Fung R, Ghiso J, Nussenzweig V, Tomlinson S (1999) Characterization of a novel trypanosome lytic factor from human serum. Infect Immun 67:1910–1916. https://doi.org/10.5897/AJB2005.000-3035
Widener J, Nielsen MJ, Shiflett A, Moestrup SK, Hajduk S (2007) Hemoglobin is a co-factor of human trypanosome lytic factor. PLoS Pathog 3:1250–1261. https://doi.org/10.1371/journal.ppat.0030129
Uzureau P, Uzureau S, Lecordier L, Fontaine F, Tebabi P, Homble F et al (2013) Mechanism of Trypanosoma brucei gambiense resistance to human serum. Nature 501:430–434. https://doi.org/10.1038/nature12516
Kieft R, Capewell P, Turner CM, Veitch NJ, MacLeod A, Hajduk S (2010) Mechanism of Trypanosoma brucei gambiense (group 1) resistance to human trypanosome lytic factor. Proc Natl Acad Sci USA 107:16137–16141. https://doi.org/10.1073/pnas.1007074107
DeJesus E, Kieft R, Albright B, Stephens NA, Hajduk SL (2013) A single amino acid substitution in the group 1 Trypanosoma brucei gambiense haptoglobin-hemoglobin receptor abolishes TLF-1 binding. PLoS Pathog 9:e1003317. https://doi.org/10.1371/journal.ppat.1003317
Lecordier L, Vanhollebeke B, Poelvoorde P, Tebabi P, Paturiaux-Hanocq F, Andris F et al (2009) C-terminal mutants of apolipoprotein L-I efficiently kill both Trypanosoma brucei brucei and Trypanosoma brucei rhodesiense. PLoS Pathog 5:e1000685. https://doi.org/10.1371/journal.ppat.1000685
Franco JR, Cecchi G, Priotto G, Paone M, Diarra A, Grout L et al (2017) Monitoring the elimination of human African trypanosomiasis: update to 2014. PLoS Negl Trop Dis 11:e0005585. https://doi.org/10.1371/journal.pntd.0005585
N'Djetchi MK, Ilboudo H, Koffi M, Kabore J, Kabore JW, Kaba D et al (2017) The study of trypanosome species circulating in domestic animals in two human African trypanosomiasis foci of Cote d'Ivoire identifies pigs and cattle as potential reservoirs of Trypanosoma brucei gambiense. PLoS Negl Trop Dis 11:e0005993. https://doi.org/10.1371/journal.pntd.0005993
Barrett MP (2018) The elimination of human African trypanosomiasis is in sight: Report from the third WHO stakeholders meeting on elimination of gambiense human African trypanosomiasis. PLoS Negl Trop Dis 12:e0006925. https://doi.org/10.1371/journal.pntd.0006925
Waiswa C, Wangoola MR (2019) Sustaining efforts of controlling zoonotic sleeping sickness in Uganda Using trypanocidal treatment and spray of cattle with deltamethrin. Vector Borne and Zoonotic Diseases 19:613–618. https://doi.org/10.1089/vbz.2018.2382
Wangoola RM, Kevin B, Acup CA, Welburn S, Waiswa C, Bugeza J (2019) Factors associated with persistence of African animal trypanosomiasis in Lango subregion, northern Uganda. Trop Ani Health Prod 51:2011–2018. https://doi.org/10.1007/s11250-019-01900-7
Herder S, Simo G, Nkinin S, Njiokou F (2002) Identification of trypanosomes in wild animals from southern Cameroon using the polymerase chain reaction (PCR). Parasite 9:345–349. https://doi.org/10.1051/parasite/2002094345
Anderson NE, Mubanga J, Fevre EM, Picozzi K, Eisler MC, Thomas R et al (2011) Characterisation of the wildlife reservoir community for human and animal trypanosomiasis in the Luangwa Valley. Zambia PLoS Negl Trop Dis 5:e1211. https://doi.org/10.1371/journal.pntd.0001211
Adams ER, Malele II, Msangi AR, Gibson WC (2006) Trypanosome identification in wild tsetse populations in Tanzania using generic primers to amplify the ribosomal RNA ITS-1 region. Acta Trop 100:103–109. https://doi.org/10.1016/j.actatropica.2006.10.002
Nok AJ, Balogun EO (2003) A bloodstream Trypanosoma congolense sialidase could be involved in anemia during experimental trypanosomiasis. J Biochem 133:725–730. https://doi.org/10.1093/jb/mvg093
Balogun EO, Balogun JB, Yusuf S, Inuwa HM, Ndams IS, Sheridan P et al (2014) Anemia amelioration by lactose infusion during trypanosomosis could be associated with erythrocytes membrane de-galactosylation. Vet Parasitol 199:259–263. https://doi.org/10.1016/j.vetpar.2013.10.013
Herbert WJ, Lumsden WH (1976) Trypanosoma brucei: a rapid "matching" method for estimating the host's parasitemia. Exp Parasitol 40:427–431. https://doi.org/10.1016/0014-4894(76)90110-7
Atawodi SE (2005) Comparative in vitro trypanocidal activities of petroleum ether, chloroform, methanol and aqueous extracts of some Nigerian savannah plants. Afr J Biotechnol 4:177–182. https://doi.org/10.5897/AJB2005.000-3035
Natala AJ, Balogun EO, Balogun JA, Inuwa HM, Nok AJ, Shiba T et al (2013) Identification and characterization of sialidase-like activity in the developmental stages of Amblyomma variegatum. J Med Entomol 50:85–93. https://doi.org/10.1603/me12152
Balogun EO, Inaoka DK, Shiba T, Kido Y, Nara T, Aoki T et al (2013) Biochemical characterization of highly active Trypanosoma brucei gambiense glycerol kinase, a promising drug target. J Biochem 154:77–84. https://doi.org/10.1093/jb/mvt037
Njiokou F, Laveissiere C, Simo G, Nkinin S, Grebaut P, Cuny G et al (2006) Wild fauna as a probable animal reservoir for Trypanosoma brucei gambiense in Cameroon. Infect Genet Evol 6:147–153. https://doi.org/10.1016/j.meegid.2005.04.003
Informal Expert Group on Gambiense HATR, Buscher P, Bart JM, Boelaert M, Bucheton B, Cecchi G et al (2018) Do cryptic reservoirs threaten gambiense-sleeping sickness elimination? Trends Parasitol. 34:197–207. https://doi.org/10.1016/j.pt.2017.11.008
Abenga J, Enwezor F, Lawani F, Osue H, Ikemereh E (2004) Trypanosome prevalence in cattle in Lere area in Kaduna State, North central Nigeria. Rev Elev Med Vet Pays Trop 57:45–48. https://doi.org/10.19182/remvt.9904
Samdi S, Fajinmi A, Kalejaye J, Wayo B, Haruna M, Yarnap J et al (2011) Prevalence of trypanosomosis in cattle at slaughter in Kaduna central Abattoir. Asian J Ani Sci 5:162–165. https://doi.org/10.3923/ajas.2011.162.165
Lema A, Maigoro M, Said M, Marwana A, Nuraddeen W (2018) Prevalence of bovine trypanasomosis in Katsina Central Abattoir. Katsina State Niger J Parasitol 39:226–229. https://doi.org/10.4314/njpar.v39i2.19
Tomlinson S, Muranjan M, Nussenzweig V, Raper J (1997) Haptoglobin-related protein and apolipoprotein AI are components of the two trypanolytic factors in human serum. Mol Biochem Parasitol 86:117–120. https://doi.org/10.1016/S0166-6851(97)02844-2
Nielsen MJ, Petersen SV, Jacobsen C, Oxvig C, Rees D, Møller HJ et al (2006) Haptoglobin-related protein is a high-affinity hemoglobin-binding plasma protein. Blood 108:2846–2849. https://doi.org/10.1182/blood-2006-05-022327
Laible G (2009) Enhancing livestock through genetic engineering–recent advances and future prospects. Comp Immunol Microbiol Infect Dis 32:123–137. https://doi.org/10.1016/j.cimid.2007.11.012
Laible G, Alonso-Gonzalez L (2009) Gene targeting from laboratory to livestock: current status and emerging concepts. Biotechnol J 4:1278–1292. https://doi.org/10.1002/biot.200900006
Shrock E, Guell M (2017) CRISPR in Animals and Animal Models. Prog Mol Biol Trans Sci 152:95–114. https://doi.org/10.1016/bs.pmbts.2017.07.010
Tait-Burkard C, Doeschl-Wilson A, McGrew MJ, Archibald AL, Sang HM, Houston RD et al (2018) Livestock 2.0—genome editing for fitter, healthier, and more productive farmed animals. Genome Biol. 19:204. https://doi.org/10.1186/s13059-018-1583-1
Balogun EO, Nok AJ, Kita K (2016) Global warming and the possible globalization of vector-borne diseases: a call for increased awareness and action. Trop Med Health 44:38. https://doi.org/10.1186/s41182-016-0039-0
Lukes J, Raper J (2010) Prophylactic antiparasitic transgenesis for human parasitic disease? Mol Ther 18:1745–1747. https://doi.org/10.1038/mt.2010.193
Acknowledgements
This paper is dedicated to our late mentor Professor Andrew Jonathan Nok who passed away in the course of this project. AJN conceived the idea of this work. Until his untimely death, he was the Principal Investigator and Center Leader of the African Center of Excellence for Neglected Tropical Diseases and Forensic Biotechnology, Ahmadu Bello University, Zaria, Nigeria (ACENTDFB-ABU). The authors acknowledge the financial support from ACENTDFB-ABU to fund this project and to support the postgraduate studies of ALA, ABY, FG, AJH. We thank the technical staff members of the Department of Biochemistry and Department of Veterinary Parasitology and Entomology, Ahmadu Bello University, Zaria, Nigeria. EOB is a FLAIR Research Fellow of The Royal Society, UK, and supported by a Global Challenge Research Fund (GCRF) Grant (No. FLR\R1\190353) through a partnership between the African Academy of Sciences and the Royal Society, UK.
Author information
Authors and Affiliations
Contributions
AJN, EOB and ALA conceptualised the study. EOB, AJN, and MNS designed the experiments. ALA carried out all the experiments. AJN, EOB and SEA supervised the work. ALA, ABY, OAA, BI, FG, AJH, MM, and EOB analysed the data. ALA and EOB wrote the manuscript. All authors reviewed the results, revised the manuscript and approved the final version.
Corresponding author
Ethics declarations
Conflict of Interest
Authors declare that there are no conflicting interests, and this manuscript is not presently submitted to another journal for publication.
Ethical Statement
Ethical approval was given by Ahmadu Bello University, Zaria, Ethical Committee on Animal Use and care (ABUCAUC). All animals used in this study were handled based on ethical guidelines on the use of animals for research purpose as stipulated by ABUCAUC.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdullahi, A.L., Balogun, E.O., Yusuf, A.B. et al. Blood of African Hedgehog Atelerix albiventris Contains 115-kDa Trypanolytic Protein that Kills Trypanosoma congolense. Acta Parasit. 65, 733–742 (2020). https://doi.org/10.2478/s11686-020-00211-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11686-020-00211-4