Abstract
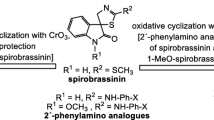
The anti-cancer properties of naturally occurring (2R, 3R)-(−)-1-methoxyspirobrassinol methyl ether (I) and their synthetic amino or piperidyl analogues II inspired us to study the synthesis of new target compounds III with a C—C bond in the 2-position of indole rather than a C—N or C—O bond (I or II respectively). The goal was achieved via electrophilic-nucleophilic 3,2-difunctionalisation of 1-methoxybrassinin (IV) in the presence of bromine and the Grignard reagent leading to the formation of cis- and trans-C—C analogues of I. Finally, the anti-cancer activities of the new compounds were measured and compared with I and II in order to show the importance of a heteroatom in the 2-substituted indole on the anti-cancer activity of spirobrassinols.
Similar content being viewed by others
References
Acheson, R. M., Hunt, P. G., Littelwood, D. M., Murrer, B. A., & Rosenberg, H. E. (1978). The synthesis, reactions, and spectra of 1-acetoxy-, 1-hydroxy-, and 1-methoxy-indoles. Journal of the Chemical Society, Perkin Transactions 1, 1978, 1117–1125. DOI: 10.1039/p19780001117.
Čurillová, M., Nakahashi, A., & Monde, K. (2007). Stereoselective synthesis of (R)-(+)-1-methoxyspirobrassinin, (2R,3R)-(−)-1-methoxy spirobrassinol methyl ether and their enantiomers or diastereoisomers. Tetrahedron Letters, 48, 8200–8204. DOI: 10.1016/j.tetlet.2007.09.080.
Entemann, C. E., Jr., & Johnson, J. R. (1933). The relative reactivity of various functional groups toward a grignard reagent. Journal of the American Chemical Society, 55, 2900–2903. DOI: 10.1021/ja01334a046.
Hanley, A. B., Parsley, K. L., Lewis, J. A., & Fenwick, G. R. (1990). Chemistry of indole glucosinolates: intermediacy of indol-3-ylmethyl isothiocyanates in the enzymic hydrolysis of indole glucosinolates. Journal of the Chemical Society, Perkin Transactions 1, 1990, 2273–2276. DOI: 10.1039/p19900002273.
Kutschy, P., & Mezencev, R. (2008). Indole phytoalexins from Brassicaceae: Synthesis and anticancer activity. In O. A. Attanasi, & D. Spinelli (Eds.), Targets in heterocyclic systems: Chemistry and properties (Vol. 12, pp. 120–148). Urbino, Italy: Italian Society of Chemistry.
Kutschy, P., Salayová, A., Čurillová, Z., Kožár, T., Mezencev, R., Mojžiš, J., Pilátová, M., Balentová, E., Pazdera, P., Sabol, M., & Zburová, M. (2009). 2-(Substituted phenyl) amino analogs of 1-methoxyspirobrassinol methyl ether: Synthesis and anticancer activity. Bioorganic & Medicinal Chemistry, 17, 3698–3712. DOI: 10.1016/j.bmc.2009.03.064.
Mezencev, R., Kutschy, P., Salayová, A., Čurillová, Z., Mojžiš, J., Pilátová, M., & McDonald, J. (2008). Anticancer properties of 2-piperidyl analogues of the natural indole phytoalexin 1-methoxyspirobrassinol. Chemotherapy, 54, 372–378. DOI: 10.1159/000152027.
Mosmann, T. (1983). Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. Journal of Immunological Methods, 65, 55–63. DOI: 10.1016/0022-1759(83)90303-4.
Pedras, M. S. C., & Zaharia, I. L. (2000). Sinalbins A and B, phytoalexins from Sinapis alba: elicitation, isolation, and synthesis. Phytochemistry, 55, 213–216. DOI: 10.1016/s0031-9422(00)00277-6.
Pedras, M. S. C., Yaya, E. E., & Glawisching, E. (2011). The phytoalexins from cultivated and wild crucifers: Chemistry and biology. Natural Product Reports, 28, 1381–1405. DOI: 10.1039/c1np00020a.
Seyferth, D. (2009). The Grignard reagents. Organometallics, 28, 1598–1605. DOI: 10.1021/om900088z.
Smith, D. H. (1999). Grignard reactions in “wet” ether. Journal of Chemical Education, 76, 1427. DOI: 10.1021/ed076p1427.
Somei, M., & Kawasaki, T. (1989). A new and simple synthesis of 1-hydroxyindole derivatives. Heterocycles, 29, 1251–1254. DOI: 10.3987/com-89-5037.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Očenáš, P., Tomášová, L., Kutschy, P. et al. Spirocyclisation of phytoalexin 1-methoxybrassinin in the presence of Grignard reagents. Chem. Pap. 67, 631–642 (2013). https://doi.org/10.2478/s11696-013-0335-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11696-013-0335-7