Abstract
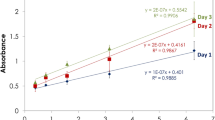
The Bacille Calmette–Guerine (BCG) vaccine, which is based on a live strain of Mycobacterium bovis BCG is widely used for the immunoprophylaxis of tuberculosis. One of the BCG vaccine’s key parameters is its сell viability (specific activity: the number of colony forming units, CFUs), which is traditionally defined by the microbiological method. In this work, the rapid and selective bioluminescent method of intracellular ATP assay is used to control the BCG vaccine’s viability at various stages of the vaccine’s production. It permits us to reduce the time required for the analysis from 28 days to 1 h. The correlation is shown between the viability of a liquid BCG vaccine measured by the microbiological method compared to one calculated using the content of intracellular ATP, as well as the correlation between the CFU value for the lyophilized BCG vaccine and the ATP content in the liquid vaccine before lyophilization.




Similar content being viewed by others
REFERENCES
Ho, M.M., Corbel, M.J., Knezevic, I., and Roumian-tzeff, M., Vaccine, 2005, vol. 23, no. 50, p. 5700.
Lundin, A., Methods Enzymol., 2000, vol. 305, p. 346.
Lomakina, G.Yu., Modestova, Yu.A., and Ugarova, N.N., Biochemistry (Moscow), 2015, vol. 80, no. 6, p. 701.
Stefanova, T., Biotechnol. Biotechnol. Equip., 2014, vol. 28, no. 3, p. 387.
Jensen, S.E., Hubrechts, P., Klein, B.M., and Haslow, K.R., Biologicals, 2008, vol. 36, no. 5, p. 308.
Ho, M.M., Markey, K., Rigsby, P., Jensen, S.E., Gairola, S., Seki, M., Castello-Branco, L.R., Lopez-Vidal, Y., Knezevic, I., and Corbel, M.J., Vaccine, 2008, vol. 26, no. 36, p. 4754.
Kolibab, K., Derrick, S.C., Jacobs, W.R., and Morris, S.L., J. Microbiol. Methods, 2012, vol. 90, no. 3, p. 245.
Soni, G.R., Guidance Document on Quality Control of Bacillus Calmette-Guerin (BCG) Vaccine, Noida: Natl. Inst. Biologicals of India, 2014, p. 3
Singh Jadaun, G.P., Kasana, H., and Malik, N., Int. J. Vaccine Res., 2016, vol. 1, no. 1, p. 4.
Jin, T.H., Qu, T., Raina, A., Alexander, P., and Tsao, E., J. Vaccines Vaccination, 2016, vol. 7, no. 1, p. 2.
Ugarova, N.N., Lomakina, G.Yu., Modestova, Yu.A., Chernikov, S.V., Vinokurova, N.V., and Gorbachev, V.Y., J. Microbiol. Methods, 2016, vol. 130, no. 1, p. 48.
Cernuschi, T., Malvolti, S., Nickels, E., and Friede, M., Vaccine, 2018, vol. 36, no. 4, p. 498.
WHO Expert Committee on Biological Standardization, report 62, Geneva, 2014, annex 3, p. 139.
Ugarova, N.N., Koksharov, M.I., and Lomakina, G.Yu., RF Patent 2420594, Byull. Izobret., 2011, no. 16.
Koksharov, M.I. and Ugarova, N.N., Protein Eng., Des. Sel., 2011, vol. 24, no. 11, p. 835.
WHO/TB/Technical Guide/77/9: In vitro Assay of BCG Products, 1977, p. 1.
Bland, J.M. and Altman, D.G., Lancet, 1986, vol. 327, p. 307.
Giavarina, G., Biochem. Med., 2015, vol. 25, no. 2, p. 141.
Funding
The work was performed as part of state assignment no. AAAA-A16-116052010081-5 of Moscow State University and was supported by AO NPO Microgen (agreement nos. 702/16 and 400/17).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by A. Muravev
Abbreviations: ATP, adenosine-5'-triphosphate; CFUs, colony-forming units.
About this article
Cite this article
Ugarova, N.N., Lomakina, G.Y., Perevyshina, T.A. et al. Controlling BCG Vaccine’s Cell Viability in the Process of Its Production by an Bioluminescent ATP Assay. Moscow Univ. Chem. Bull. 74, 191–197 (2019). https://doi.org/10.3103/S0027131419040084
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0027131419040084