Abstract
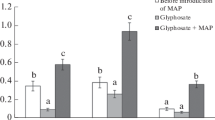
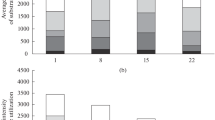
Under laboratory conditions, the decomposition of glyphosate with the formation of aminomethylphosphonic acid (AMPA) and its effect on the total abundance of bacteria and fungi as well as the number of copies of genes encoding the enzymes C–P lyase of α-proteobacteria (phnJ), acid and alkaline phosphatase (phoC and phoD) and Fe protein of nitrogenase (nifH) in agro-sod-podzolic soil (Epialbic Retisol) were determined. It was shown that when applying glyphosate in recommended doses (5–10 mg/kg), only 5–7% of the introduced herbicide were detected after 14 days, but when the dose was increased to 100 mg/kg, this value increased up to 23%. Decreasing the rate of the herbicide degradation was observed only during the first week of incubation and was accompanied by a decrease in the number of copies of the phoC, phoD, and nifH genes and an increase in the abundance of fungi. The obtained results indicate that glyphosate was mainly degraded by means of C–P bond breaking and the formation of phosphates, and also suggest possible inhibition of the nitrogen fixation process. It is shown that at an application dose of glyphosate of 100 mg/kg may lead to the accumulation of AMPA, the first metabolite of the herbicide degradation pathway, formed after the C–N bond break. Bioassay using wheat showed that when applying glyphosate at a dose of 100 mg/kg, an inhibition of plant development was observed: the length of the roots and the biomass of the shoots reduced by 60 and 20% compared to the control, respectively. Based on the data obtained, it was proposed to use the reduction in the content of copies of the phoC gene and the increase in the number of copies of ITS rRNA as indicators of the predominant decomposition of glyphosate through the sarcosine pathway. The decrease in the number of copies of ITS rRNA gene by 40% or more can be used as an indicator of the possibility of AMPA accumulation during glyphosate degradation.


Similar content being viewed by others
REFERENCES
Zhelezova, A.D., Tkhakakhova, A.K., Yaroslavtseva, N.V., et al., Microbiological parameters of aggregates in typical chernozems of long-term field experiments, Eurasian Soil Sci., 2017, vol. 50, no. 6, pp. 701–708.
Kulikova, N.A., Zhelezova, A.D., Voropanov, M.G., et al., Monoammonium phosphate effects on glyphosate in soils: mobilization, phytotoxicity, and alteration of the microbial community, Eurasian Soil Sci., 2020, vol. 53, no. 6, pp. 787–798.
Sviridov, A.V., Shushkova, T.V., Ermakova, I.T., et al., Microbial degradation of glyphosate herbicides (review), Appl. Biochem. Microbiol., 2015, vol. 51, no. 2, pp. 188–196.
Adelowo, F.E., Olu-Arotiowa, O.A., and Amuda, O.S., Biodegradation of glyphosate by fungi species, Adv. Biosci. Bioeng., 2014, vol. 2, no. 1.
Allegrini, M., Gomez, E.V., and Zabaloy, M.C., Repeated glyphosate exposure induces shifts in nitrifying communities and metabolism of phenylpropanoids, Soil Biol. Biochem., 2017, vol. 105, pp. 206–215.
Araujo, A.S.F., Monteiro, R.T.R., and Abarkeli, R.B., Effect of glyphosate on the microbial activity of two Brazilian soils, Chemosphere, 2003, vol. 52, no. 5, pp. 799–804.
Atherton, F.R., Hall, M.J., Hassall, C.H., et al., Antibacterial activity and mechanism of action of phosphonopeptides based on aminomethylphosphonic acid, Antimicrob. Agents Chemother., 1982, vol. 22, no. 4.
Banks, M.L., Kennedy, A.C., Kremer, R.J., and Eivazi, F., Soil microbial community response to surfactants and herbicides in two soils, Appl. Soil Ecol., 2014, vol. 74, pp. 12–20.
Battaglin, W.A., Meyer, M.T., Kuivila, K.M., and Dietze, J.E., Glyphosate and its degradation product AMPA occur frequently and widely in U.S. soils, surface water, groundwater, and precipitation, J. Am. Water Res. Assoc., 2014, vol. 50, no. 2.
Blackshaw, R.E. and Harker, K.N., Wheat, field pea, and canola response to glyphosate and AMPA soil residue, Weed Technol., 2016, vol. 30, no. 4.
Borggaard, O.K. and Gimsing, L., Fate of glyphosate insoil and the possibility of leaching to ground and surfacewaters: a review, Pest Manage. Sci., 2008, vol. 64, no. 4, pp. 441–456.
Bott, S., Tesfamariam, T., Kania, A., et al., Phytotoxicity of glyphosate soil residues re-mobilised by phosphate fertilization, Plant Soil, 2011, vol. 342, pp. 249–263.
Chen, M.X., Cao, Z.Y., Jiang, Y., and Zhu, Z.W., Direct determination of glyphosate and its major metabolite, aminomethylphosphonic acid, in fruits and vegetables by mixed-mode hydrophilic interaction/weak anion-exchange liquid chromatography coupled with electrospray tandem mass spectrometry, J. Chromatogr. A, 2013, vol. 1272, pp. 90–99.
Chen, X., Jiang, N., Chen, Z., et al., Response of soil phoD phosphatase gene to long-term combined applications of chemical fertilizers and organic materials, Appl. Soil Ecol., 2017, vol. 119, pp. 197–204.
Cherni, A.E., Trabelsi, D., Chebil, S., et al., Effect of glyphosate on enzymatic activities, Rhizobiaceae and totalbacterial communities in an agricultural Tunisian soil, Water, Air Soil Pollut., 2015, vol. 226, art. 145.
Claassens, A., Ros, M.T., Van Zwieten, L., et al., Soil-borne glyphosate residue thresholds for wheat seedling metabolite profiles and fungal root endophyte colonization are lower than for biomass production in a sandy soil, Plant Soil, 2019, vol. 438, pp. 393–404.
Druart, C., Delhomme, O., de Vaufleury, A., et al., Optimization of extraction procedure and chromatographic separation of glyphosate, glufosinate and aminomethylphosphonic acid in soil, Anal. Bioanal. Chem., 2011, vol. 399, no. 4, pp. 1725–1732.
Erban, T., Stehlik, V., Sopko, B., et al., The different behaviors of glyphosate and AMPA in compost-amended soil, Chemosphere, 2018, vol. 207, pp. 78–83.
Ermakova, I.T., Shushkova, T.V., Sviridov, A.V., et al., Organophosphonates utilization by soil strains of Ochrobactrum anthropi and Achromobacter sp, Arch. Microbiol., 2017, vol. 199, pp. 665–675. https://sun9-24.userapi.com/c206824/v206824187/e76ea/UL_tg0jEHCk.jpg
Fan, L., Feng, Yu., Weaver, D.B., et al., Glyphosate effects on symbiotic nitrogen fixation in glyphosate-resistant soybean, Appl. Soil. Ecol., 2017, vol. 21, pp. 11–19.
Fierer, N., Jackson, J.A., Vilgalys, R., and Jacksson, R.B., Assessment of soil microbial community structure by use of taxon-specific quantitative PCR assays, Appl. Environ. Microbiol., 2005, vol. 71, no. 7, pp. 4117–4120.
Fraser, T.D., Lynch, D.H., Gaiero, J., et al., Quantification of bacterial non-specific acid (phoC) and alkaline (phoD) phosphatase genes in bulk and rhizosphere soilfrom organically managed soybean fields, Appl. Soil Ecol., 2017, vol. 111, pp. 48–56.
Karasali, H., Pavlidis, G., and Marousopoulou, A., Investigation of the presence of glyphosate and its major metabolite AMPA in Greek soils, Environ. Sci. Pollut. Res., 2019, vol. 26, pp. 36308–36321. https://doi.org/10.1007/s11356-019-06523-x
Kryuchkova, Y.V., Burygin, G.L., Gogoleva, N.E., et al., Isolation and characterization of a glyphosate-degrading rhizosphere strain, Enterobacter cloacae K7, Microbiol. Res., 2014, vol. 169, no. 1.
Lancaster, S.H., Hollister, E.B., Senseman, S.A., and Gentry, T.J., Effects of repeated glyphosate applications onsoil microbial community composition and the mineralization of glyphosate, Pest Manag. Sci., 2010, vol. 66, no. 1, pp. 59–64.
Liu, C.-M., McLean, P.A., Sookdeo, C.C., and Cannon, F.C., Degradation of the herbicide glyphosate by members of the family Rhizobiaceae, Appl. Environ. Microbiol., 1991, vol. 57, no. 6, pp. 1799–1804.
Miloševiã, N.A. and Govedarica, M.M., Effect of herbicides on microbiological properties of soil, Proc. Nat. Sci. –Matica Srp., 2002, vol. 102, pp. 5–21.
Newman, M.M., Hoilett, N., Lorenz, N., et al., Glyphosate effects on soil rhizosphere-associated bacterial communities, Sci. Total Environ., 2016, vol. 543, pp. 155–160.
Niemeyer, J.C., de Santo, F.B., Guerra, N., et al., Do recommended doses of glyphosate-based herbicides affect soil invertebrates? Field and laboratory screening tests to risk assessment, Chemosphere, 2018, vol. 198, pp. 154–160.
Okada, E., Costa, J.L., and Bedmar, F., Adsorption and mobility of glyphosate in different soils under no-till andconventional tillage, Geoderma, 2016, vol. 263, pp. 78–85.
Okada, E., Costa, J.L., and Bedmar, F., Glyphosate dissipation in different soils under no-till and conventional tillage, Pedosphere, 2019, vol. 29, no. 6.
Pipke, R. and Amrhein, N., Carbon-phosphorus lyase activity in permeabilized cells of Arthrobacter sp. GLP-1, FEBS Lett., 1988, vol. 236, no. 1, pp. 135–138.
Pizzul, L., Castillo, M.D.P., and Stenström, J., Degradation of glyphosate and other pesticides by ligninolytic enzymes, Biodegradation, 2009, vol. 20, art. 751.
Poly, F., Ranjard, L., Nazaret, S., et al., Comparison of nifH gene pools in soils and soil microenvironments with contrasting properties, Appl. Environ. Microbiol., 2001, vol. 67, no. 5, pp. 2255–2262.
Ragot, S.A., Kertesz, M.A., and Bünemann, E.K., phoD alkaline phosphatase gene diversity in soil, Appl. Environ. Microbiol., 2015, vol. 81, pp. 7281–7289.
Reddy, K.N., Rimando, A.M., and Duke, S.O., Aminomethylphosphonic acid, a metabolite of glyphosate, causesinjury in glyphosate-treated, glyphosate-resistant soybean, J. Agric. Food Chem., 2004, vol. 52, no. 16, pp. 5139–5143.
Santos, A. and Flores, M., Effects of glyphosate on nitrogen fixation of free-living heterotrophic bacteria, Lett. Appl. Mlcrobiol., 1995, vol. 20, no. 6, pp. 349–352.
Shehata, A., Kühnert, M., Haufe, S., and Krüger, M., Neutralization of the antimicrobial effect of glyphosate by humic acid in vitro,Chemosphere, 2014, vol. 104, pp. 258–261.
Singh, H., Singh, N.B., Singh, A., and Hussain, I., Exogenous application of salicylic acid to alleviate glyphosate stress in Solanum lycopersicum,Int. J. Veg. Sci., 2017, vol. 23, no. 9.
Singh, H., Singh, N.B., Singh, A., et al., Physiologicaland biochemical roles of nitric oxide against toxicity produced by glyphosate herbicide in Pisum sativum,Russ. J. Plant Physiol., 2017, vol. 64, pp. 518–524.
Soares, C., Pereira, R., Spormann, S., and Fidalgo, F., Is soil contamination by a glyphosate commercial formulation truly harmless to non-target plants? Evaluation of oxidative damage and antioxidant responses in tomato, Environ. Pollut., 2019, vol. 247, pp. 256–265.
Soares, C., Spormann, S., and Fidalgo, F., Salicylic acid improves the performance of the enzymatic antioxidant system of barley exposed to glyphosate, Free Radicals Biol. Med., 2018, vol. 120, suppl. 1, p. S157.
Tang, F.H.M., Jeffries, T.C., Vervoort, R.V., et al., Microcosm experiments and kinetic modeling of glyphosate biodegradation in soils and sediment, Sci. Total Environ., 2019, vol. 658, pp. 105–115.
Tejada, M., Evution of soil biological propertiesafter addition of glyphosate, diflufenican and glyphosate + diflufenican herbicides, Chemosphere, 2009, vol. 76, no. 3, pp. 365–373.
Yao, M., Henny, C., and Maresca, J.A., Freshwater bacteria release methane as a by-product of phosphorus acquisition, Appl. Environ. Microbiol., 2016, vol. 82, pp. 6994–7003.
Zabaloy, M.C., Zanini, G.P., Bianchinotti, V., et al., Herbicides in the soil environment: linkage between bioavailability and microbial ecology, in Herbicides, Theory and Applications, London: InTechOpen, 2011.
Zhan, H., Feng, Y., Fan, X., and Chen, S., Recent advances in glyphosate biodegradation, Appl. Microbiol. Biotechnol., 2018, vol. 102, no. 12, pp. 5033–5043.
Funding
The work was supported by the Russian Science Foundation, project no. 19-16-00053 (development of molecular genetic analysis methods) as a part of government task (CITIS no. 116020110002-8) (collection and characterization of soil samples).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflicts of interest.Statement on the welfare of humans or animals. This article does not contain any studies involving animals performed by any of the authors.
Additional information
Translated by A. Bulaev
About this article
Cite this article
Kulikova, N.A., Zhelezova, A.D., Filippova, O.I. et al. The Degradation of Glyphosate and Its Effect on the Microbial Community of Agro-Sod–Podzolic Soil under Short-Term Model Experiment Conditions. Moscow Univ. Soil Sci. Bull. 75, 138–145 (2020). https://doi.org/10.3103/S0147687420030035
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0147687420030035