Abstract
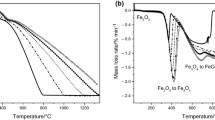
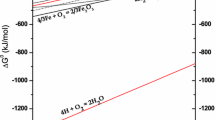
Hydrogen is mainly used during reduction annealing of iron powder produced by spraying of liquid metal by water. Chemical aspects of this process have been simulated using TERRA 6.3 software. In particular, Fe–O–H thermodynamic system has been analyzed a in wide range of hydrogen temperatures and flow rates. It follows from the performed analysis that the main impurity compounds of the sprayed powder are not iron hydrates but Fe2O3 oxide. However, it cannot exist in hydrogen environment and is converted into Fe3O4 oxide at low temperature. Therefore, the main reaction of iron reduction will be the reaction Fe3O4 + 4H2 = 3Fe + 4H2O, which terminates at 910°C. It has been demonstrated that this temperature can be significantly decreased accompanied by the equally significant hydrogen flow rate. Consideration of this factor can be useful for optimization of powder annealing mode.
Similar content being viewed by others
REFERENCES
Girshov, V.L., Kotov, S.A., and Cemenko, V.N., Sovremennye tekhnologii v poroshkovoi metallurgii (Modern Technologies in Powder Metallurgy), St. Petersburg: Izd-vo Politekh. Univ., 2010.
Trusov, B.G., Baza dannykh i programmnyi kompleks TERRA, redaktsiya 6.3 (Database and Program Complex TERRA, Version 6.3), Moscow: Mosk. Gos. Tekh. Univ. im. Baumana, 2013.
The rusting process (information service). http:// www.docbrown.info/page03/Reactivitya.htm.
Spreitzer, D. and Schenk, J., Reduction of iron oxides with hydrogen—A review, Steel Res. Int., 2019, vol. 90, no. 10, p. 1900108. https://doi.org/10.1002/srin.201900108
Zieliński, J., Zglinicka, I., Znak, L., and Kaszkur, Z., Reduction of Fe2O3 with hydrogen, Appl. Catal., A, 2010, vol. 381, nos. 1–2, pp. 191–196. https://doi.org/10.1016/j.apcata.2010.04.003
Berdnikov, V.I. and Gudim, Yu.A., Chemical reactions in carbon gasification processes, Steel Transl., 2019, vol. 49, pp. 593–600. https://doi.org/10.3103/S096709121909002X
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by I. Moshkin
About this article
Cite this article
Berdnikov, V.I., Gudim, Y.A. Chemical Reactions during Iron Reduction from Oxides by Hydrogen. Steel Transl. 52, 52–53 (2022). https://doi.org/10.3103/S0967091222010065
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0967091222010065