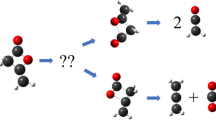
Abstract—Quantum-mechanical ab initio calculations are applied to study the growth mechanism of polycyclic aromatic hydrocarbons via reactions of 1- and 2‑naphthyl with propyne (methylacetylene) and allene (1,2‑propadiene). It was shown that stabilized isomers С13Н11 are mostly formed at temperatures <1000 K. At temperatures above 1000 K, the yield of bimolecular three-ring products C13H10 + H and C12H8 + CH3 reaches 70%. The total reaction rate constants are in the range of 10–12–10–11 cm3 molecule–1 s–1 at temperatures of 1000–2500 K.


Similar content being viewed by others

REFERENCES
X. Yang and D. H. Zhang, “Dynamical Resonances in the Fluorine Atom Reaction with the Hydrogen Molecule,” Acc. Chem. Res. 41, 981 (2008). https://doi.org/10.1021/ar700258g
K. N. Urness, Q. Guan, A. Golan, J. W. Daily, et al., “Pyrolysis of Furan in a Microreactor,” J. Chem. Phys. 139, 124305 (2013). https://doi.org/10.1063/1.4821600
M. Frenklach and H. Wang, “Detailed Modeling of Soot Particle Nucleation and Growth,” Proc. Combust. Inst. 23, 1559 (1991). https://doi.org/10.1016/S0082-0784(06)80426-1
L. Zhao, R. I. Kaiser, B. Xu, U. Ablikim, et al., “Low-Temperature Formation of Polycyclic Aromatic Hydrocarbons in Titan’s Atmosphere,” Nat. Astron. 2, 973 (2018). https://doi.org/10.1038/s41550-018-0585-y
A. M. Mebel, A. Landera, and R. I. Kaiser, “Formation Mechanisms of Naphthalene and Indene: from the Interstellar Medium to Combustion Flames,” J. Phys. Chem. A 121, 901 (2017). https://doi.org/10.1021/acs.jpca.6b09735
A. D. Becke, “Density-Functional Thermochemistry III. The Role of Exact Exchange,” J. Chem. Phys. 98, 5648 (1993). https://doi.org/10.1063/1.464913
P. L. Fast, M. L. Sánchez, and D. G. Truhlar, “Multi-Coefficient Gaussian-3 Method for Calculating Potential Energy Surfaces,” Chem. Phys. Lett. 306, 407 (1999). https://doi.org/10.1016/S0009-2614(99)00493-5
Y. Georgievskii, J. A. Miller, M. P. Burke, and S. J. Klippenstein, “Reformulation and Solution of the Master Equation for Multiple-Well Chemical Reactions,” J. Phys. Chem. A 117, 12146 (2013). https://doi.org/10.1021/jp4060704
L. Zhao, M. Prendergast, R. I. Kaiser, et al., “How to Add a Five-Membered Ring to Polycyclic Aromatic Hydrocarbons (PAHs)-Molecular Mass Growth of the 2-Naphthyl Radical (C10H7) to Benzindenes (C13H10) as a Case Study,” Phys. Chem. Chem. Phys. 21, 16737 (2019). https://doi.org/10.1039/C9CP02930C
Funding
This work was supported by the Ministry of Science and Higher Education of the Russian Federation, project no. 14.Y26.31.0020.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by A. Kazantsev
About this article
Cite this article
Oleinikov, A.D., Mebel, A.M. & Azyazov, V.N. Kinetics of Reactions of 1- and 2‑Naphthyl with Propyne and Allene. Bull. Lebedev Phys. Inst. 47, 97–100 (2020). https://doi.org/10.3103/S1068335620030057
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1068335620030057