Abstract
Aims/hypothesis
Besides serving as a traditional inflammatory marker, C-reactive protein (CRP) is closely associated with the development of obesity, diabetes and cardiovascular diseases as a metabolic and inflammatory marker. We hypothesise that CRP protein directly participates in the regulation of energy and glucose metabolism rather than just being a surrogate marker, and that genetic deficiency of CRP will lead to resistance to obesity and insulin resistance.
Methods
Crp gene deletion was achieved by transcription activator-like effector nuclease (TALEN) technology in rats. The Crp knockout animals were placed on either a standard chow diet or a high-fat diet. Phenotypic changes in body weight, glucose metabolism, insulin sensitivity, energy expenditure and inflammation condition were examined. The central impact of CRP deficiency on leptin and insulin hypothalamic signalling, as well as glucose homeostasis, were examined via intracerebral ventricular delivery of leptin and CRP plus glucose clamp studies in the wild-type and Crp knockout rats.
Results
CRP deficiency led to a significant reduction in weight gain and food intake, elevated energy expenditure and improved insulin sensitivity after exposure to high-fat diet. Glucose clamp studies revealed enhanced hepatic insulin signalling and actions. Deficiency of CRP enhanced and prolonged the weight-reducing effect of central injected leptin and promoted the central and peripheral roles of leptin. By contrast, reinstatement of CRP into the hypothalamus of the knockout rats attenuated the effects of central leptin signalling on insulin sensitivity and peripheral glucose metabolism.
Conclusions/interpretation
This study represents the first line of genetic evidence that CRP is not merely a surrogate blood marker for inflammation and metabolic syndromes but directly regulates energy balance, body weight, insulin sensitivity and glucose homeostasis through direct regulation of leptin’s central effect and hypothalamic signalling.
Graphical abstract

Similar content being viewed by others

Introduction
Chronic low-grade inflammation is known to contribute to the occurrence and development of insulin resistance and type 2 diabetes [1]. A large number of inflammatory factors have been identified as predictors of insulin resistance, type 2 diabetes and other metabolic diseases [2, 3], among which C-reactive protein (CRP) is a reliable and widely used clinical biomarker [4, 5]. As a member of the pentraxin protein family, CRP is mainly synthesised and secreted by the liver and released into circulation in response to inflammation [6]. Numerous in vivo and in vitro studies have demonstrated that CRP is associated with insulin resistance and metabolic diseases such as obesity, hepatic steatosis, cardiovascular disease and type 2 diabetes [7,8,9]. Despite these strong association findings from clinical studies, the critical question remains unanswered: does CRP functionally participate in the regulation of glucose homeostasis, inflammation and energy metabolism or is it merely a surrogate bystander?
Leptin is an adipokine that is synthesised and released by adipose tissue and plays an indispensable role in the control of body weight and food intake [10]. Leptin resistance is an important feature in the development of obesity, insulin resistance and type 2 diabetes in humans [11]. Early studies (including our own) have shed some light on the potential role of CRP in regulating leptin’s functions [11]. For instance, human C-reactive protein (hCRP) can bind to leptin to block leptin signalling and impair its function, which in turn confers leptin resistance [12]. In ob/ob mice, subcutaneous administration of hCRP attenuated the effects of exogenous leptin on food intake and body weight [12]. Furthermore, elevation of hCRP within the central nervous system (CNS) could negate the actions of leptin on energy balance [13].
The physiological actions of both insulin and leptin can be negatively influenced by chronic inflammation, in which CRP is intimately involved; it is therefore critical to define the direct and precise impact of CRP on leptin-regulated energy balance and metabolism at the genetic level under in vivo conditions. Although studies on a CRP-targeting mouse model have revealed its protective role in acute inflammation [14], mouse CRP is not regarded as an ideal system for investigating the actions of hCRP, particularly for the study in metabolism and cardiovascular functions. Therefore, research on the metabolic roles of CRP has been hampered by the lack of appropriate CRP-deficient animal models. On this note, it is encouraging to see the report that manipulation of circulating rat CRP levels with antibodies has successfully simulated the actions of hCRP in the cardiovascular system [15], implying that rat CRP can function in a fashion similar to that of hCRP. Herein, we report the creation of a rat Crp gene-targeting model to investigate the impact of CRP deficiency on energy balance, inflammation and insulin sensitivity. With this Crp knockout (KO) rat model, we would be able to define, at the genetic level, whether and how CRP directly regulates energy balance, insulin sensitivity and glucose homeostasis.
Methods
Animals
Crp KO rats were generated from Cyagen Biosciences and then housed in the Animal Experimental Centre of Chongqing Medical University. Crp KO rats on a Sprague Dawley background were through transcription activator-like effector nuclease (TALEN) technology (CYAGEN, Guangzhou, China). Briefly, the analysis of rat Crp exonic and intronic sequences led to the design of the location of a gene deletion (exon 1). We used this to construct the TALEN plasmid (sequences for lead mRNAs: TALEN-1-L: TCACGATAAGCTTCTCTC, TALEN-1-R: TATAATAGCTCCTACCTT). Nuclear injection of the in vitro transcribed mRNA resulted in the birth of the F0 generation of positive rats, which were crossed with the wild-type (WT) to produce the heterozygotic F1 generation. Heterozygous F1 rats were mated with heterozygous F1 rats to obtain homozygous Crp KO rats or heterozygous F2 or WT littermates. The phenotype was confirmed through sequencing. Animals were housed in a temperature- and humidity-controlled facility with 12 h light/dark cycles with ad libitum access to water and food. All animals used were based on their corresponding genotypes, once their genotypes were identified, and they were randomly assigned to either the NCD- or HFD-fed groups, or randomly assigned to various treatment groups. To construct a high-fat diet (HFD)-induced insulin resistance model, eight-week-old male WT and Crp KO rats were fed either a normal chow diet (NCD) or an HFD (45% fat; Medicine, Jiangsu, China) for 12 weeks. For the age-related metabolic study, eight-week-old male WT and Crp KO rats were fed an HFD for 16 months. Animal experimental procedures were approved by the Animal Experimentation Ethics Committee, Chongqing Medical University.
Cannulation and indwelling catheters
For the intracerebroventricular (ICV) study, 7 days prior to the in vivo experiments, rats were anaesthetised and fixed in a stereotaxic apparatus. Indwelling catheters were stereotaxically implanted into the third cerebral ventricle (V3). The correct cannula placement into V3 was confirmed by testing the drinking response to angiotensin II as described previously [16]. For the euglycaemic–hyperinsulinaemic clamp (EHC) study, 7 days after stereotaxic surgery the rats were anaesthetised with intraperitoneal pentobarbital (50 mg/kg) and catheters were inserted into the right internal jugular vein and left carotid artery.
ICV infusion protocols
To investigate the dose-dependent effects of ICV CRP and leptin infusion on glucose metabolism, different concentrations of CRP (22.5, 11.25 and 2.25 μg/h) and leptin (2.5, 0.5 μg/h and 15, 0.5 ng/h; R&D Systems, MN, USA) were continuously infused into the ICV area through the ICV catheter during the GTT. Blood glucose was measured at the indicated time points. For subsequent experiments we infused CRP (3.75 μg/h) and leptin (0.83 μg/h) into the ICV area during the EHC.
GTT, ITT and EHC
GTTs and ITTs were conducted as reported previously [17]. For the EHC study, rats were restricted to ~230.12 kJ of food the day before the EHC to keep the identical nutritional status during the EHC. After a 12 h fast, EHCs were performed in conscious, unrestrained rats as described previously [18]. Briefly, a continuous infusion of HPLC–purified [3-H3]glucose (PerkinElmer, MA, USA; 2.22 × 105 Bq bolus, 7.4 × 103 Bq/min) was initiated at 0 min and maintained throughout the experiment. The EHCs were started at time 90 min after [3-H3]glucose infusion. Somatostatin (3 μg kg−1 min−1) was infused intravenously together with insulin (1.2 mU kg−1 min−1) to inhibit endogenous insulin secretion. A 25% glucose solution was started at time 90 min and adjusted every 10 min to maintain blood glucose at basal levels. For ICV infusion protocols, at time −120 min, ICV infusions of PBS or recombinant rat leptin (0.83 μg/h, R&D Systems) and/or CRP (3.75 μg/h, R&D systems) were initiated and maintained until the end of the EHC. Blood samples were collected at the indicated time points for insulin, NEFA, and glucose-specific activity measurements. To assess insulin-mediated glucose uptake in fat and muscle tissues, a 2-deoxy-d [3H]glucose (2-DG, Amersham, Los Angeles, CA, USA) was administered as a bolus (1.1 × 106 Bq) 45 min before the end of clamp experiments. Blood samples (50 μl) were taken at 5, 15, 30 and 45 min after the injection to determine the tracer disappearance curve. At the end of the EHC, the rats were anaesthetised and tissue samples were obtained and freeze-clamped in situ with aluminium tongs precooled in liquid nitrogen and stored at −80°C.
Measurement of hepatic triacylglycerol level
Liver tissues were homogenised and centrifuged at 12,000 g at 4°C for 15 min. The supernatant was collected and the content of liver triacylglycerol was determined according to the instructions (Triglyceride Colorimetric Assay Kit, Cayman Chemical, USA).
Metabolic analyses and indirect calorimetry in conscious rats
Body weight was measured daily in rats aged 8–20 weeks for the HFD-induced insulin resistance study and from age 6–18 months for the age-related metabolic study. Food intake, locomotor tolerance and rectal temperature were measured at the indicated time points and duration. Rectal temperature was measured with an electronic thermometer. For cold adaptation, rats were placed at 4°C for 3 h, and the skin temperature was recorded using an infrared Thermoviewer (FOTRIC 220, FOTRIC, Shanghai, China). Energy expenditure was calculated by 24 h \( \dot{V}{\mathrm{O}}_2 \) and \( \dot{V}\mathrm{C}{\mathrm{O}}_2 \), as described previously.
Long-term ICV leptin treatment
To determine the dose-dependent effects of ICV leptin on food intake, eight-week-old male WT and Crp KO rats were fed an HFD for 12 weeks and were treated with ICV leptin injection twice daily for 6 days. To determine the dosage for ICV leptin injection, different doses of leptin (0.5, 2.5, 7.5 and 15 μg) were injected into the ICV area of rats. Food intake was measured.
Immunohistochemistry
Rat hypothalamic tissues were collected, fixed in 4% paraformaldehyde, and paraffin-embedded following ICV leptin treatment. After incubation with a primary antibody against signal transducer and activator of transcription 3 (STAT3) (#12640, 1:400, Cell Signaling Technology, Boston, MA, USA) or phospho-STAT3 (#9145, 1:400, Cell Signaling Technology, Boston, MA, USA) and a goat anti-rabbit secondary antibody detection kit (Zsbio, Beijing, China), the tissue sections were visualised with a confocal microscope (Nikon A1+R, Tokyo, Japan).
Analysis of mRNA and proteins
Real-time quantitative PCR was performed as described previously using β-actin as an internal control gene [19]. Primer sequences are listed in electronic supplementary material (ESM) Table 1. Protein expression was analysed by western blot, as indicated previously [20]. Basic information on all the antibodies used in this study is listed in ESM Table 2. All antibodies were diluted with antibody diluent (Beyotime Biotechnology, Shanghai, China).
Animal PET and data acquisition
Positron emission tomography (PET)–computed tomography (CT) scans were performed on rats on a Super Nova PET/CTS (PINGSENG Medical Technology, Kunshan, Jiangsu Province, China). Fat volume analysis is based on the grey scale value of fat (CT value) lower than other organs and muscles. Fat was screened and separated according to different threshold values of grey scale value. Cruiser software (version 1.5.0, PINGSENG Medical Technology) was used to capture the images, and the collection parameters were set as 60 kV tube pressure, 600 μA tube flow, DSD (the vertical distance from the source focus to the detector) 510 mm and FOV (field of view) 110 mm. After image acquisition, recon software (recon 1.5.0, PINGSENG Medical Technology) was used for reconstruction. This process was carried out by professionals.
Statistical analysis
Data are expressed as mean ± standard error of the mean. For comparing two groups, two-tailed unpaired Student’s t test was used for unpaired data. The analysis of the GTT and ITT at each time point was performed using Student’s t test for paired data. We used WT littermates of the Crp KO rats for the GTT and ITT. Where necessary, variance analysis (ANOVA) was used, followed by Tukey’s post hoc test for multiple comparisons of means. This information is provided in the legends of the respective figures. A p value <0.05 was considered to be statistically significant.
Results
The impact of CRP deficiency on energy balance
To investigate the physiological effect of CRP deficiency on energy homeostasis, Crp KO rats were generated using TALEN technology (see Methods). CRP protein expression was not detectable in the livers of the Crp KO rats, and the circulating CRP levels were also nil compared with the expression in the WT littermates. Quantitative PCR analysis also revealed complete knockout of Crp expression in the fat, muscle, and pancreas (ESM Fig. 1a–c). While examining the growth of the Crp KO rats fed on NCD, we found no discernible difference in body weight, fat composition, and food intake (Fig. 1a–g). In addition, the rectal temperature (measured at either room temperature or 4°C) and thermal images showed no significant differences between the two genotypes (ESM Fig. 2a, b).
Crp KO rats displayed reduced gain in body weight, fat mass, food intake and blood pressure. Eight-week-old male WT and Crp KO rats were fed an NCD or HFD for 12 weeks. The Crp KO protects the rats from the weight gain as evident in the body weight growth curve (a) and the cumulative body weight (b) during high-fat feeding. A micro-CT scan showed significant reduction in fat composition (c, d) and fat mass (but not in lean mass) (e). Under the HFD regimen, Crp KO also led to decreased mean daily food intake (f) and cumulative food intake (g) as well as the systolic blood pressure (h) or diastolic blood pressure (i). Data are expressed as the mean ± SEM, *p<0.05, **p<0.01 for Crp KO rats compared with WT rats (unpaired Student’s t test was used for data). ATV, adipose tissue volume
Under an HFD regimen, the body weight, volume of fat and energy balance phenotypes showed divergence between the mutant and WT rats. Accordingly, the body weight, fat mass and food intake of Crp KO rats were significantly reduced while the lean mass was not significantly changed (Fig. 1a–f) compared with WT rats. The mean daily food intake and cumulative food intake were also lower in the HFD-fed KO rats than in the WT controls (Fig. 1f, g). Interestingly, the Crp KO rats almost completely resisted the elevation of systolic and diastolic blood pressure induced by HFD (Fig. 1h, i); however, such phenotype appeared to accompany the attenuated weight gain of the Crp KO rats (ESM Fig. 3a, b). Whether this phenotype was only secondary to the differences in weight gain or a result of CRP’s direct participation in the regulation of blood pressure remains to be investigated.
Upon further dissecting the impact of Crp deficiency on energy homeostasis under HFD conditions, we found that the Crp KO rats showed increased energy expenditure. Consistent with this observation, the rectal temperature was higher in the Crp KO rats (Fig. 2a), and thermal imaging analysis showed that the Crp KO rats had a higher temperature in interscapular brown adipose tissue than the WT rats (Fig. 2b). Further exposure to a cold environment at 4°C also revealed elevated temperature in the mutant rats (Fig. 2c). In a 24 h metabolic cage study, \( \dot{V}{\mathrm{O}}_2 \) (Fig. 2d, e) and \( \dot{V}\mathrm{C}{\mathrm{O}}_2 \) (Fig. 2f) were increased relative to those in the HFD-fed WT rats. The HFD-fed KO rats also displayed a reduced respiratory exchange ratio (RER, \( \dot{V}{\mathrm{O}}_2 \)/\( \dot{V}\mathrm{C}{\mathrm{O}}_2 \)) (total or during the day, Fig. 2g, h), implying an increased tendency towards burning off fat. Interestingly, locomotor tolerance was higher in the NCD-fed KO rats than that in the WT rats, while there was no significant difference between the two groups of HFD-fed rats (Fig. 2i, j). Taken together, these results demonstrate that CRP deficiency promotes the host’s resistance to obesity and development of high blood pressure, and gives rise to elevated energy expenditure and enhanced locomotor activity.
Crp KO rats displayed elevated energy expenditure. Under high-fat feeding, the KO rats displayed increased rectal temperature (a). When placed at 4°C, the thermal images (b) and rectal temperature (c) all indicated elevated heat generation in the KO rats. In metabolic cage studies, the 24 h \( \dot{V}{\mathrm{O}}_2 \) (d, e) and 24 h \( \dot{V}\mathrm{C}{\mathrm{O}}_2 \) (f) were all significantly increased, while the RER ratios (g, h) decreased, in the KO rats while fed with HFD. In the locomotor test, both the running distance (i) and running time (j) were higher in \( \dot{V}{\mathrm{O}}_2 \) the KO rats than those in the WT controls fed NCD. Data are expressed as the mean ± SEM (n=8–10/group). *p<0.05, **p<0.01 vs NCD-WT or HFD-WT as shown (unpaired Student’s t test was used for data)
CRP deficiency ameliorates HFD-induced insulin resistance
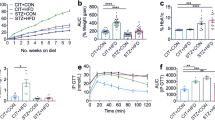
Elevated circulating CRP levels have been clinically associated with insulin resistance and development of type 2 diabetes. Other than a modestly decreased level of fasting blood insulin and triacylglycerol, there was no discernible difference in a series of metabolic indications, such as fasting blood glucose, NEFA, and total cholesterol between the Crp KO and WT rats while they were maintained on NCD (ESM Table 3). Under the same condition, GTT and ITT in conscious and unrestrained rats also did not show differences between the two genotypes (Fig. 3a, b). However, when challenged with HFD, the Crp KO rats displayed better GTT and ITT curves than the WT rats (Fig. 3a, b). We then performed an EHC protocol to investigate the impact of Crp deletion on glucose metabolic kinetics in the livers of both genotypes under both NCD and HFD conditions (Fig. 3c; basic blood metabolic parameters during the EHC provided in ESM Table 4). Under both the NCD and HFD regimens, the glucose infusion rate (GIR) (Fig. 3d, e) and glucose disposal rate (GDR) (Fig. 3f) were greater, while hepatic glucose production (HGP) was lower (Fig. 3g, h), in the Crp KO rats than those in the WT rats, indicating the enhancement of global insulin sensitivity in the mutant rats. In addition, glucose uptake was markedly higher in the skeletal muscle tissues (Fig. 3i), but not in the adipose tissues (Fig. 3j), of the HFD-fed Crp KO rats when compared with those in the WT rats. Consistent with these results from the clamp studies in vivo, quantitative PCR and western blot analysis showed that the mRNA and protein expression of hepatic PEPCK was significantly decreased, and insulin-stimulated phosphorylation of hepatic InsR and Akt was more pronounced, in the Crp KO rats than those in the WT controls (Fig. 3k, l). We also measured the expression of PEPCK in the fat tissue, which is involved in gluconeogenesis, under HFD conditions and found no significant difference between the WT and the KO rats (ESM Fig. 4a). To confirm whether Crp KO has an impact on lipid accumulation in the liver, we examined the triacylglycerol content in liver. The levels of triacylglycerol in the liver were significantly lower in HFD-fed Crp KO rats than in the rats of the HFD-fed WT group (ESM Fig. 4b). In addition, we performed hepatic section staining and found lipid droplets were numerous and augmentative in the livers of the HFD group. However, lipid droplets obviously decreased in HFD-fed Crp KO rats (ESM Fig. 4c). Importantly, measurement of cytokines revealed that Crp KO rats completely resisted HFD-induced elevation of circulating TNF-α levels (ESM Fig. 5), indicating that CRP plays an intimate role in promoting inflammatory response. Taken together, these observations provide strong genetic evidence that CRP is directly involved in the regulation of hepatic and skeletal muscular insulin sensitivity as well as chronic inflammation, rather than just being a surrogate circulating marker of these two physiological events.
Crp KO rats are resistant to HFD-induced insulin resistance. Eight-week-old male WT and Crp KO rats were fed an NCD or HFD for 12 weeks. The GTT (a) and ITT (b) curves are shown; **p<0.01 vs HFD-WT at specific time points of test, as shown. Illustration of the experimental procedure and EHC protocol (c). Crp deficiency enhanced insulin sensitivity as evident from the elevated GIR shown in the GIR time course (d), the cumulative GIR (e) and the GDR (f), in both NCD- and HFD-fed animals. In the EHC protocol, Crp KO also led to pronounced suppression of HGP (g, h), and further enhanced glucose uptake in the skeletal muscle tissue (i), but not in the adipose tissue (j). Determination of hepatic expression of Pepck mRNA (k). Western blot assays of hepatic expression of PEPCK protein, insulin-stimulated phosphorylation of InsR and Akt and densitometric quantification (l). Data are expressed as the mean ± SEM (n=8–10/group). In (e–l) †p<0.05, ††p<0.01 vs NCD-WT; *p<0.05, **p<0.01 vs HFD-WT (a, b: paired t test, statistical tests are paired at each time point; e–l unpaired Student’s t test)
The impact of CRP deficiency on the central action of leptin in rats
Some of the metabolic and energy balance phenotypes described above echo some of our previous findings that showed that hCRP could interact with human leptin and neutralise the function of leptin [12]. To explore the hypothesis that such leptin-associated mechanisms underlie the observed phenotypes, we performed ICV infusion of leptin in unrestrained rats, during which we evaluated glucose metabolism and insulin sensitivity with EHC studies using a tracer dilution methodology (Fig. 4a). In Crp KO rats maintained on an NCD diet, infusion of ICV leptin resulted in significantly higher GIR than that in the WT rats (Fig. 4b, c). Systemic CRP deficiency also significantly promoted leptin-initiated inhibition of HGP (Fig. 4d, e) as well as elevation of GDR (Fig. 4f). In addition, Crp KO also promoted central leptin-mediated glucose uptake in skeletal muscle but not in adipose tissue (Fig. 4g, h). Similar findings were discovered when the protocols were performed on the rats maintained on HFD. Compared with HFD-fed WT rats, ICV delivery of leptin in HFD-fed Crp KO rats resulted in a marked increase of GIR (Fig. 5a, b) and GDR (Fig. 5e) as well as a reduction in HGP by 46% (Fig. 5c, d). Again, centrally delivered leptin generated more pronounced glucose uptake in the skeletal muscle, but not the adipose tissue, in the mutant rats than in the WT control rats (Fig. 5f, g). The signalling pathways leading to the activation of hypothalamic STAT3 and Akt are critical for leptin-mediated energy metabolism [21,22,23]. Indeed, western blot analysis revealed that the phosphorylation of hypothalamic STAT3 and Akt was significantly elevated in the HFD-fed Crp KO rats compared with the HFD-fed WT rats (Fig. 5h). Combined together, these results strongly support the concept that systemic CRP deficiency promoted the effect of central leptin on hepatic and skeletal muscle glucose metabolism, and enhanced central leptin-stimulated STAT3/Akt signalling, particularly under HFD-induced obesity and insulin resistance conditions.
The effect of central leptin injection on glucose metabolism in normal diet-fed WT and Crp KO rats. Eight-week-old male WT and Crp KO rats were fed NCD for 12 weeks. The experimental procedure and clamp protocol (a). Crp deficiency elevated leptin-stimulated GIR as evident in the GIR time course (b) during the EHC and the cumulative GIR (c). Crp KO further decreased HGP (d, e), while increasing GDR (f). Crp deficiency enhanced leptin-stimulated glucose uptake in the skeletal muscle tissue (g), but not in the adipose tissue (h). Data are expressed as the mean ± SEM (n=8/group). *p<0.05, **p<0.01 vs NCD-Crp KO-Leptin or NCD-WT-PBS, as shown (two-way ANOVA); PBS was used as a control of leptin
The effect of central leptin infusion on glucose metabolism in HFD-fed WT and Crp KO rats. Eight-week-old male WT and Crp KO rats were fed HFD for 12 weeks. The experimental procedure and clamp protocol were the same as shown in Fig. 4a. Crp deficiency elevated leptin-stimulated GIR as evident in the GIR time course (a) during the EHC and the cumulative GIR (b). Crp KO further decreased HGP (c, d), while increasing GDR (e). Crp deficiency enhanced leptin-stimulated glucose uptake in the skeletal muscle tissue (f), but not in the adipose tissue (g). The effects of ICV leptin on STAT3 and Akt phosphorylation in hypothalamus from NCD- and HFD-fed WT and Crp KO rats (h). Data are expressed as the mean ± SEM (n=6–8/group). *p<0.05, **p<0.01 vs HFD-Crp KO-PBS or HFD-WT-PBS, as shown (a–g, two-way ANOVA; h, unpaired Student’s t test); PBS was used as a control of leptin
Blockade of the effects of CNS leptin action by restoration CRP level in the CNS
The findings thus far have shown that CRP deficiency enhanced the functions of hypothalamic leptin. We hypothesised that the restoration of CRP level in the CNS would neutralise leptin signalling and its physiological effects. To test this hypothesis, CRP and/or leptin were infused into the ICV region of NCD-fed Crp KO or WT rats, and an EHC protocol was performed to assess the changes in hepatic glucose kinetics in vivo (Fig. 6a). ICV infusion of leptin alone in the Crp KO rats predictably elevated peripheral GIR and GDR by 12% and 6%, respectively, relative to the ICV infusion of PBS in the KO or WT rats (Fig. 6b, c). Following the re-introduction of CRP through ICV infusion in Crp KO rats, GIR and GDR were significantly attenuated by 40% and 15%, respectively, compared with the parallel vehicle infusion alone in the KO rats, re-affirming the regulation of glucose metabolism by CRP. Importantly, ICV co-infusion of CRP and leptin overcame the inhibitory effects of the re-introduction of CRP on GIR and GDR (Fig. 6b, c). In a parallel analysis, ICV restoration of rat CRP in Crp KO rats stimulated HGP, relative to ICV delivery of PBS (Fig. 6d, e), while at the same time, it decreased glucose uptake in skeletal muscle (but not in the adipose tissue) (Fig. 6f, g). Importantly, in both cases, ICV co-infusion of both CRP and leptin completely blunted the effect of CRP restoration (Fig. 6b–f). Central delivery of rat CRP also blocked leptin signalling in the hypothalamus of Crp KO rats. ICV infusion of CRP alone already significantly inhibited Akt phosphorylation in the hypothalamus compared with ICV infusion of the vehicle in the Crp KO rats (Fig. 6h). Although ICV infusion of leptin alone predictably increased Akt phosphorylation, co-infusion of CRP with leptin in the Crp KO rats markedly inhibited leptin-stimulated Akt phosphorylation (Fig. 6h). Thus, restoration of CRP within the CNS disrupted glucose homeostasis and promoted hepatic and skeletal muscle insulin resistance. These deleterious effects could be overcome by co-infusion with leptin, supporting the notion that CRP-initiated regulation of metabolism is mediated at least in part through blocking leptin’s actions.
The effect of CRP re-constitution in the hypothalamus of the Crp KO rats on leptin-regulated glucose metabolism in vivo. The experimental procedure and clamp protocol: eight-week-old WT and Crp KO rats were fed an NCD for 12 weeks. An ICV catheter was implanted on rats on day 1. The internal jugular vein and carotid artery cannulations were performed on day 7, and the EHC and ICV leptin and/or ICV CRP were performed on day 10, as indicated in the Methods (a). Leptin-stimulated cumulative GIR (b) and GDR (c) were increased in the CRP-deficient rats, while co-infusion with rat CRP blunted leptin’s effect. Similarly, CNS infusion of leptin led to suppression in HGP (d, e) in the Crp KO rats but not in the WT rats, which was neutralised by the CNS co-infusion of CRP. CNS infusion of leptin stimulated glucose uptake in skeletal muscle tissue (f), but not in the adipose tissue (g) in the Crp KO rats. Determination of Akt phosphorylation in the hypothalamus (h). Data are expressed as the mean ± SEM (n=8/group). *p<0.05, **p<0.01 vs Crp KO-PBS, Crp KO-Leptin, Crp KO-CRP or Crp KO-Leptin-CRP, as shown (a–g, two-way ANOVA; h, unpaired Student’s t test)
Effect of continuous ICV injection of leptin on energy homeostasis in HFD-fed rats
The findings described above prompted us to further interrogate the theory that the presence of CRP constitutes part of the mechanisms underlying leptin resistance, and that, if so, CRP deficiency would allow a persistent and prolonged weight-reducing effect following exogenous leptin injection. To test this concept, we examined the food intake and body weight following a standard consecutive central injection of leptin under the HFD regimen. Such a protocol would typically generate an immediate drop in food intake in the WT animals, followed by a rapid rebound in food consumption, which was also evident in our experiment (Fig. 7a). ICV injection of leptin twice daily for 6 days in HFD-fed Crp KO rats resulted in a more pronounced reduction in food intake from day 1 to day 4, relative to that in the WT rats (Fig. 7a). Although food intake also began to show a modest increase in the KO rats starting from day 4, the anorexic effect was still sharply lower than that in the WT controls (Fig. 7a). Similar patterns of changes were also observed in body weight from day 2 to day 6 (Fig. 7b).
The effects of repeated ICV leptin treatment on energy homeostasis in HFD-fed Crp KO rats. Eight-week-old male WT and Crp KO rats were fed an HFD for 12 weeks and were treated with ICV leptin twice daily for 6 days, as indicated in the Methods. Daily leptin injection led to greater and prolonged reduction in food intake (a) and body weight (b) in the KO rats than in the WT controls. Determination of the expression levels of Pomc, Agrp, and Npy mRNA in the hypothalamus (c). Determination of the level of STAT3 phosphorylation in the hypothalamus by western blot assays (d). Immunohistochemical staining for STAT3 phosphorylation in the hypothalamus (e); original magnification, ×100 or enlarged ×200 (enlargement shown by box), scale bars 200 μm, or 100 μm in enlarged images. 3V, third ventricle and ARC, arcuate nucleus. Data are expressed as the mean ± SEM (n=4–5/group for the western blot and immunostaining experiments). *p<0.05, **p<0.01 vs HFD-WT, WT-PBS or WT, ††p<0.01 vs WT-Leptin. (c, two-way ANOVA; a–b, d and e, unpaired Student’s t test ); PBS was used as a control of leptin
Consistent with these physiological observations, quantitative PCR analysis revealed a sharp increase in the hypothalamic expression of Pomc in the Crp KO rats following ICV leptin treatment (Fig. 7c); however, hypothalamic expression of Agrp and Npy was not significantly different between the two genotypes. In addition, western blot analysis showed that consecutive ICV leptin injection persistently elevated STAT3 phosphorylation in the hypothalamus of the Crp KO rats relative to that in the control rats (Fig. 7d), a result also confirmed by immunohistochemical staining of phosphorylated STAT3 (Fig. 7e). Thus, Crp deficiency profoundly ameliorated leptin resistance, and allowed prolonged and sustained anorexic and weight-reducing effects. Elevated blood CRP is directly involved in leptin resistance as well as diminished insulin sensitivity.
The effect of Crp deletion on body weight and insulin sensitivity during ageing
The incidence of metabolic syndromes increases with ageing, both of which have been shown to be positively associated with blood CRP levels [24]. By inference, Crp deficiency is expected to have long-lasting metabolic benefits, particularly during ageing. WT and Crp KO rats were placed under NCD or HFD diet regimens for 18 months. From 8 months to 18 months, the gain in body weight induced by HFD was persistently lower (Fig. 8a, b), while rectal temperature was higher (Fig. 8c), throughout the observed ageing period in the Crp KO than in the WT rats. Interestingly, during the same process, HFD-induced increase of systolic and diastolic blood pressure remained attenuated (Fig. 8d, e) in the Crp KO rats. In glucose metabolism, GTT and ITT tests showed that the improvement in glucose metabolism and insulin sensitivity lasted for at least 12 months, as indicated by the improved profiles in the Crp KO rats (Fig. 8f, g at 8 months of age, and h, i at 12 months of age). Combined together, these findings unequivocally demonstrate that CRP has a long-term, persistent regulatory impact on body weight energy expenditure and glucose metabolism, as well as blood pressure.
Effect of Crp deficiency on body weight, insulin sensitivity and other metabolic variables during ageing. In a long-term study (particularly on HFD for 72 weeks), the KO rats consistently gained less weight than their WT controls as reflected in the body weight curve (a) and cumulative body weight (b). The rectal temperature (c) was also higher in the HFD-fed KO rats than in the controls throughout the observation; meanwhile, the systolic (d) and diastolic (e) blood pressure readings were also significantly lower in the KO animals than in the controls. In the GTTs performed at 8 months (f) or 12 months (g), or the parallel ITTs (h; 8 months) and (i; 12 months), the HFD-fed KO animals displayed significantly enhanced GTT and ITT profiles. Data are expressed as the mean ± SEM (n=8–10/group). *p<0.05, **p<0.01 vs HFD-WT (a–e, unpaired Student’s t test; f–i, paired t test, statistical tests are paired at each time point)
Discussion
Previous genetic studies have revealed no null mutations and very few variations in the amino acid sequences of CRP [25,26,27], which implies that CRP has important physiological functions that are critical to the survival and well-being of hosts. Even though mouse CRP is not necessarily equivalent to hCRP in terms of functions, Crp KO in mice demonstrated that circulating CRP protects the host from pulmonary damage following microbial infection [28, 29]. Indeed, we also found that Crp KO rats were much more susceptible to inflammatory cell infiltration and lung damage than the WT controls following the acute treatment of lipopolysaccharide (LPS) (data not shown), which indicates that CRP is not merely a marker of acute inflammation [30,31,32].
CRP in mice is not an acute phase reactant and shows a very mild response to LPS stimulation [33,34,35,36]. Although Crp KO mice have been developed [37,38,39], they have limited use in the study of CRP functions in metabolic and cardiovascular diseases. For example, Crp deletion in mice did not affect the development of atherosclerotic plaques. In fact, these pitfalls in developing a mouse model of Crp deletion propelled the development of transgenic mice harbouring the hCRP gene (CRP) for investigating autoimmune and cardiovascular diseases [36, 40, 41]. Interestingly, interference of rat CRP functions by exogenous injection of specific antibodies did decrease atherosclerotic development [42], implying that rats are a better model of human physiology than mice in this regard [43]. However, it is difficult to use this approach to stably affect CRP expression to allow the study of metabolic phenotypes. Therefore, the development of Crp KO rats can serve as a genetic model for studying the physiology of CRP, especially in relation to metabolic diseases, insulin resistance and energy balance.
Abundant clinical studies in the past decade have demonstrated strong positive correlations between blood CRP levels and adiposity, insulin resistance and type 2 diabetes [27, 44]. However, the question of whether CRP actively participates in the regulation of metabolism still requires definitive genetic proof. The results presented in this study demonstrate that systemic Crp deficiency provided the rats with resistance against HFD-induced gain in body weight, insulin resistance and chronic inflammation, and also significantly promoted energy expenditure and insulin-regulated glucose metabolism. These phenotypes mirrored CRP elevation in human obesity and type 2 diabetes, and fully support the hypothesis that CRP is a direct regulator of metabolism rather than just a metabolic disease marker.
The metabolic phenotypes that result from systemic CRP deficiency are mediated, at least in part, through the regulation of the physiological functions of leptin that are indispensable for controlling a series of physiological functions, such as energy balance, and glucose and fat metabolism [45, 46]. Previous research has shown that hCRP can interact with human leptin and negatively impact the physiological functions of human leptin in the CNS of ob/ob mice [13, 47]. However, these studies were carried out in a heterologous system and the duration of observation was short, and as a result, it was difficult to assess the effects of hCRP on leptin signalling in the CNS or in the periphery. The genetic model of Crp KO rats allowed us to investigate the metabolic function of CRP over an extended period, including the ageing process. For example, repeated ICV injection of leptin in the Crp KO rats resulted in sustained weight loss and reduction of food intake, which was in sharp contrast to the diminution of leptin’s anorexic actions as well as sharp rebound of body weight in the WT rats. Importantly, reinstatement of CRP in the hypothalamus of Crp KO rats negated the effects of leptin on energy balance as well as glucose metabolism, and essentially re-instated the phenotypes of CRP-induced leptin resistance. The lack of CRP expression further promoted leptin-stimulated STAT3 phosphorylation and the expression of pro-opiomelanocortin (POMC), an anorexic peptide, in the hypothalamus. Combined together, these results provide strong genetic evidence that CRP directly participates in the regulation of energy balance by negatively influencing leptin’s actions.
Largely mediated via its central receptors at the hypothalamus, leptin’s important roles in regulating hepatic glucose fluxes, insulin sensitivity and lipid metabolism have been well documented [48, 49]. By inference, the negative impact of CRP on the functions of leptin should counter its actions in metabolism. Indeed, following the central delivery of leptin, glucose clamp analysis showed that Crp deficiency led to an increase in GIR, reduced HGP and elevated glucose uptake in the muscles. Consistent with these observations, hepatic insulin signalling was also significantly enhanced. Chronic inflammatory condition, a contributing factor to insulin resistance [50,51,52], was significantly ameliorated in the Crp KO rats, as indicated by the drop in blood TNF-α levels. Earlier studies have shown that rat CRP could promote inflammation by directly binding to FcγR on macrophages, thus inhibiting the production of IL-10 while increasing the synthesis of TNF-α and IL-12 [53,54,55] . We also noted that the protective effects on metabolic phenotypes was long term, indicating that the regulation of CRP on body weight and insulin sensitivity can last through the ageing process. However, this protective effect did diminish, although it was still statistically significant, as the KO animals aged beyond 12 months. This is reminiscent of the Il6 KO model in which the mice were protected from the metabolic syndromes at a young age, but this effect did not last during the ageing process [56, 57]. It is likely that as the animal becomes older, the build-up of fat and the changes of various hormones (e.g. the decline of testosterone), will also play prominent roles in the regulation of metabolism. Thus, a rise in blood CRP levels will be detrimental to insulin sensitivity and glucose homeostasis, as a result of the combined effects on leptin signalling and inflammation, which eventually leads to type 2 diabetes.
In summary, we have for the first time generated a rat Crp KO model to provide genetic evidence that CRP directly regulates energy balance, chronic inflammation, blood pressure, glucose metabolism and insulin sensitivity. Some of these pathophysiological effects were achieved, at least in part, through controlling inflammation and negating leptin’s physiological functions. These findings unequivocally support the concept that elevation of blood CRP levels in people with obesity and type 2 diabetes is not merely a surrogate marker. Reduction of circulating CRP levels or CRP-induced leptin resistance may serve as a promising therapeutic strategy for the treatment of obesity and type 2 diabetes.
Data availability
Data related to this manuscript are available on request from the authors.
Abbreviations
- CNS:
-
Central nervous system
- CRP:
-
C-reactive protein
- EHC:
-
Euglycaemic–hyperinsulinaemic clamp
- GIR:
-
Glucose infusion rate
- GDR:
-
Glucose disposal rate
- hCRP:
-
Human C-reactive protein
- HFD:
-
High-fat diet
- HGP:
-
Hepatic glucose production
- ICV:
-
Intracerebroventricular
- InsR:
-
Insulin receptor
- KO:
-
Knockout
- LPS:
-
Lipopolysaccharide
- NCD:
-
Normal chow diet
- RER:
-
\( \dot{V}{\mathrm{O}}_2 \)/\( \dot{V}\mathrm{C}{\mathrm{O}}_2 \)
- STAT3:
-
Signal transducer and activator of transcription 3
- TALEN:
-
Transcription activator-like effector nuclease
- WT:
-
Wild-type
References
Fernández-Real JM, Ricart W (2003) Insulin resistance and chronic cardiovascular inflammatory syndrome. Endocr Rev 24(3):278–301. https://doi.org/10.1210/er.2002-0010
Schmidt MI, Duncan BB, Sharrett AR et al (1999) Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): a cohort study. Lancet 353(9165):1649–1652. https://doi.org/10.1016/s0140-6736(99)01046-6
Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM (2001) C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 286(3):327–334. https://doi.org/10.1001/jama.286.3.327
Juszczak A, Pavić T, Vučković F et al (2019) Plasma Fucosylated Glycans and C-Reactive Protein as Biomarkers of HNF1A-MODY in Young Adult-Onset Nonautoimmune Diabetes. Diabetes Care 42(1):17–26. https://doi.org/10.2337/dc18-0422
Medina G, Gutiérrez-Moreno AL, Vera-Lastra O, Saavedra MA, Jara LJ (2011) Prevalence of metabolic syndrome in primary antiphospholipid syndrome patients. Autoimmun Rev 10(4):214–217. https://doi.org/10.1016/j.autrev.2010.10.004
Pepys MB, Baltz ML (1983) Acute phase proteins with special reference to C-reactive protein and related proteins (pentaxins) and serum amyloid A protein. Adv Immunol 34:141–212. https://doi.org/10.1016/s0065-2776(08)60379-x
Yudkin JS, Stehouwer CDA, Emeis JJ, Coppack SW (1999) C-reactive protein in healthy subjects : associations with obesity, insulin resistance, and endothelial dysfunction : a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol 19(4):972–978. https://doi.org/10.1016/s0049-3848(99)00061-4
Kotronen A, Yki-Järvinen H (2008) Fatty liver: a novel component of the metabolic syndrome. Arterioscler Thromb Vasc Biol 28(1):27–38. https://doi.org/10.1161/atvbaha.107.147538
Xi L, Xiao C, Bandsma RH, Naples M, Adeli K, Lewis GF (2011) C-reactive protein impairs hepatic insulin sensitivity and insulin signaling in rats: role of mitogen-activated protein kinases. Hepatology 53(1):127–135. https://doi.org/10.1002/hep.24011
Procopio C, Andreozzi F, Laratta E et al (2009) Leptin-stimulated endothelial nitric-oxide synthase via an adenosine 5′-monophosphate-activated protein kinase/Akt signaling pathway is attenuated by interaction with C-reactive protein. Endocrinology 150(8):3584–3593. https://doi.org/10.1210/en.2008-0921
Maffei M, Halaas J, Ravussin E et al (1995) Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med 1(11):1155–1161. https://doi.org/10.1038/nm1195-1155
Chen K, Li F, Li J et al (2006) Induction of leptin resistance through direct interaction of C-reactive protein with leptin. Nat Med 12(4):425–432. https://doi.org/10.1038/nm1372
Li J, Wei D, McCrory MA et al (2016) Human C-reactive protein impedes entry of leptin into the CNS and attenuates its physiological actions in the CNS. Biochem J 473(9):1215–1224. https://doi.org/10.1042/bj20151282
Szalai AJ, Nataf S, Hu XZ, Barnum SR (2002) Experimental allergic encephalomyelitis is inhibited in transgenic mice expressing human C-reactive protein. J Immunol 168(11):5792–5797. https://doi.org/10.4049/jimmunol.168.11.5792
Guan H, Wang P, Hui R, Edin ML, Zeldin DC, Wang DW (2009) Adeno-associated virus-mediated human C-reactive protein gene delivery causes endothelial dysfunction and hypertension in rats. Clin Chem 55(2):274–284. https://doi.org/10.1373/clinchem.2008.115857
Yang M, Zhang Z, Wang C et al (2012) Nesfatin-1 action in the brain increases insulin sensitivity through Akt/AMPK/TORC2 pathway in diet-induced insulin resistance. Diabetes 61(8):1959–1968. https://doi.org/10.2337/db11-1755
Lin Y, Liang Z, He L et al (2019) Gut ghrelin regulates hepatic glucose production and insulin signaling via a gut-brain-liver pathway. Cell Commun Signal 17(1):8. https://doi.org/10.1186/s12964-019-0321-y
Yang M, Wang J, Wu S et al (2017) Duodenal GLP-1 signaling regulates hepatic glucose production through a PKC-δ-dependent neurocircuitry. Cell Death Dis 8(2):e2609. https://doi.org/10.1038/cddis.2017.28
Wu T, Zhang Q, Wu S et al (2019) CILP-2 is a novel secreted protein and associated with insulin resistance. J Mol Cell Biol 11(12):1083–1094. https://doi.org/10.1093/jmcb/mjz016
Yang M, Liang Z, Yang M et al (2019) Role of bone morphogenetic protein-9 in the regulation of glucose and lipid metabolism. FASEB J 33(9):10077–10088. https://doi.org/10.1096/fj.201802544RR
Buettner C, Pocai A, Muse ED, Etgen AM, Myers MG Jr, Rossetti L (2006) Critical role of STAT3 in leptin’s metabolic actions. Cell Metab 4(1):49–60. https://doi.org/10.1016/j.cmet.2006.04.014
Zhao AZ, Huan JN, Gupta S, Pal R, Sahu A (2002) A phosphatidylinositol 3-kinase phosphodiesterase 3B-cyclic AMP pathway in hypothalamic action of leptin on feeding. Nat Neurosci 5(8):727–728. https://doi.org/10.1038/nn885
Bates SH, Stearns WH, Dundon TA et al (2003) STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature 421(6925):856–859. https://doi.org/10.1038/nature01388
Ridker PM (2007) Inflammatory biomarkers and risks of myocardial infarction, stroke, diabetes, and total mortality: implications for longevity. Nutr Rev 65(12 Pt 2):S253–S259. https://doi.org/10.1111/j.1753-4887.2007.tb00372.x
Hage FG, Szalai AJ (2007) C-reactive protein gene polymorphisms, C-reactive protein blood levels, and cardiovascular disease risk. J Am Coll Cardiol 50(12):1115–1122. https://doi.org/10.1016/j.jacc.2007.06.012
Miller DT, Zee RY, Suk Danik J et al (2005) Association of common CRP gene variants with CRP levels and cardiovascular events. Ann Hum Genet 69(Pt 6):623–638. https://doi.org/10.1111/j.1529-8817.2005.00210.x
Ridker PM, Pare G, Parker A et al (2008) Loci related to metabolic-syndrome pathways including LEPR, HNF1A, IL6R, and GCKR associate with plasma C-reactive protein: the Women’s Genome Health Study. Am J Hum Genet 82(5):1185–1192. https://doi.org/10.1016/j.ajhg.2008.03.015
Perez L (2019) Acute phase protein response to viral infection and vaccination. Arch Biochem Biophys 671:196–202. https://doi.org/10.1016/j.abb.2019.07.013
Szalai AJ, Briles DE, Volanakis JE (1996) Role of complement in C-reactive-protein-mediated protection of mice from Streptococcus pneumoniae. Infect Immun 64(11):4850–4853. https://doi.org/10.1128/iai.64.11.4850-4853.1996
Ridker PM (2016) From C-Reactive Protein to Interleukin-6 to Interleukin-1: Moving Upstream To Identify Novel Targets for Atheroprotection. Circ Res 118(1):145–156. https://doi.org/10.1161/circresaha.115.306656
Kapur R, Kim M, Shanmugabhavananthan S, Liu J, Li Y, Semple JW (2015) C-reactive protein enhances murine antibody-mediated transfusion-related acute lung injury. Blood 126(25):2747–2751. https://doi.org/10.1182/blood-2015-09-672592
Marques-Vidal P, Schmid R, Bochud M et al (2012) Adipocytokines, hepatic and inflammatory biomarkers and incidence of type 2 diabetes. the CoLaus study. PLoS One 7(12):e51768. https://doi.org/10.1371/journal.pone.0051768
Torzewski J (2005) C-reactive protein and atherogenesis: new insights from established animal models. Am J Pathol 167(4):923–925. https://doi.org/10.1016/s0002-9440(10)61182-0
Szalai AJ, McCrory MA (2002) Varied biologic functions of C-reactive protein: lessons learned from transgenic mice. Immunol Res 26(1–3):279–287. https://doi.org/10.1385/ir:26:1-3:279
Trion A, de Maat MP, Jukema JW et al (2005) No effect of C-reactive protein on early atherosclerosis development in apolipoprotein E*3-leiden/human C-reactive protein transgenic mice. Arterioscler Thromb Vasc Biol 25(8):1635–1640. https://doi.org/10.1161/01.ATV.0000171992.36710.1e
Hirschfield GM, Gallimore JR, Kahan MC et al (2005) Transgenic human C-reactive protein is not proatherogenic in apolipoprotein E-deficient mice. Proc Natl Acad Sci U S A 102(23):8309–8314. https://doi.org/10.1073/pnas.0503202102
Jones NR, Pegues MA, McCrory MA et al (2011) Collagen-induced arthritis is exacerbated in C-reactive protein-deficient mice. Arthritis Rheum 63(9):2641–2650. https://doi.org/10.1002/art.30444
Teupser D, Weber O, Rao TN, Sass K, Thiery J, Fehling HJ (2011) No reduction of atherosclerosis in C-reactive protein (CRP)-deficient mice. J Biol Chem 286(8):6272–6279. https://doi.org/10.1074/jbc.M110.161414
Zhang L, Shen ZY, Wang K et al (2019) C-reactive protein exacerbates epithelial-mesenchymal transition through Wnt/β-catenin and ERK signaling in streptozocin-induced diabetic nephropathy. FASEB J 33(5):6551–6563. https://doi.org/10.1096/fj.201801865RR
Szalai AJ, McCrory MA, Xing D et al (2014) Inhibiting C-reactive protein for the treatment of cardiovascular disease: promising evidence from rodent models. Mediat Inflamm 2014:353614. https://doi.org/10.1155/2014/353614
Wright TT, Jimenez RV, Morgan TE et al (2015) Hepatic but Not CNS-Expressed Human C-Reactive Protein Inhibits Experimental Autoimmune Encephalomyelitis in Transgenic Mice. Autoimmune Dis 2015:640171. https://doi.org/10.1155/2015/640171
Griselli M, Herbert J, Hutchinson WL et al (1999) C-reactive protein and complement are important mediators of tissue damage in acute myocardial infarction. J Exp Med 190(12):1733–1740. https://doi.org/10.1084/jem.190.12.1733
Suzuki H, Ito Y, Shinohara M et al (2016) Gene targeting of the transcription factor Mohawk in rats causes heterotopic ossification of Achilles tendon via failed tenogenesis. Proc Natl Acad Sci U S A 113(28):7840–7845. https://doi.org/10.1073/pnas.1522054113
Freeman DJ, Norrie J, Caslake MJ et al (2002) C-reactive protein is an independent predictor of risk for the development of diabetes in the West of Scotland Coronary Prevention Study. Diabetes 51(5):1596–1600. https://doi.org/10.2337/diabetes.51.5.1596
Dube MG, Torto R, Kalra SP (2008) Increased leptin expression selectively in the hypothalamus suppresses inflammatory markers CRP and IL-6 in leptin-deficient diabetic obese mice. Peptides 29(4):593–598. https://doi.org/10.1016/j.peptides.2008.01.001
Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM (1994) Positional cloning of the mouse obese gene and its human homologue. Nature 372(6505):425–432. https://doi.org/10.1038/372425a0
Hutchinson WL, Coll AP, Gallimore JR, Tennent GA, Pepys MB (2007) Is leptin an important physiological regulator of CRP? Nat Med 13(1):17–18; author reply 19–21. https://doi.org/10.1038/nm0107-17
Allison MB, Pan W, MacKenzie A et al (2018) Defining the Transcriptional Targets of Leptin Reveals a Role for Atf3 in Leptin Action. Diabetes 67(6):1093–1104. https://doi.org/10.2337/db17-1395
Vanpatten S, Karkanias GB, Rossetti L, Cohen DE (2004) Intracerebroventricular leptin regulates hepatic cholesterol metabolism. Biochem J 379(Pt 2):229–233. https://doi.org/10.1042/bj20040134
Sierksma A, Patel H, Ouchi N et al (2004) Effect of moderate alcohol consumption on adiponectin, tumor necrosis factor-alpha, and insulin sensitivity. Diabetes Care 27(1):184–189. https://doi.org/10.2337/diacare.27.1.184
Banerjee A, Singh J (2020) Remodeling adipose tissue inflammasome for type 2 diabetes mellitus treatment: Current perspective and translational strategies. Bioeng Transl Med 5(2):e10150
Lee SC, Pu YB, Thomas GN et al (2000) Tumor necrosis factor alpha gene G-308A polymorphism in the metabolic syndrome. Metab Clin Exp 49(8):1021–1024. https://doi.org/10.1053/meta.2000.7704
Ballou SP, Lozanski G (1992) Induction of inflammatory cytokine release from cultured human monocytes by C-reactive protein. Cytokine 4(5):361–368. https://doi.org/10.1016/1043-4666(92)90079-7
Mold C, Rodriguez W, Rodic-Polic B, Du Clos TW (2002) C-reactive protein mediates protection from lipopolysaccharide through interactions with Fc gamma R. J Immunol 169(12):7019–7025. https://doi.org/10.4049/jimmunol.169.12.7019
Singh U, Devaraj S, Dasu MR, Ciobanu D, Reusch J, Jialal I (2006) C-reactive protein decreases interleukin-10 secretion in activated human monocyte-derived macrophages via inhibition of cyclic AMP production. Arterioscler Thromb Vasc Biol 26(11):2469–2475. https://doi.org/10.1161/01.ATV.0000241572.05292.fb
Wallenius V, Wallenius K, Ahrén B et al (2002) Interleukin-6-deficient mice develop mature-onset obesity. Nat Med 8(1):75–79. https://doi.org/10.1038/nm0102-75
Matthews VB, Allen TL, Risis S et al (2010) Interleukin-6-deficient mice develop hepatic inflammation and systemic insulin resistance. Diabetologia 53(11):2431–2441. https://doi.org/10.1007/s00125-010-1865-y
Acknowledgements
Some of the data were presented as an abstract at the 56th EASD Annual Meeting in 2020.
Authors’ relationships and activities
The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Funding
This work was supported by research grants from the National Natural Science Foundation of China (81800755 to MY, 81630021 to AZZ); the national Key R&D Program of China (2018YFa0800600 to AZZ); the Key research and development program of Guangdong Province for ‘innovative drug creation’ (2019B020201015 to FL); and the Guangdong Innovative Research Team Program (2016ZT06Y432 to AZZ and FHL).
Author information
Authors and Affiliations
Contributions
MY and SQ designed and performed experiments, analysed results, and wrote and edited the manuscript. YH, TW and LL performed and analysed the results obtained from the EHC experiments in addition to editing the manuscript. ND and FL helped with interpretation and analyses of the data from Crp KO rats, and edited the manuscript. AZZ and GY conceived the study, designed and performed experiments, analysed results, and wrote and edited the manuscript. All authors read and approved the final manuscript. AZZ and GY are the guarantors of this work.
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM
(PDF 844 kb)
Rights and permissions
About this article
Cite this article
Yang, M., Qiu, S., He, Y. et al. Genetic ablation of C-reactive protein gene confers resistance to obesity and insulin resistance in rats. Diabetologia 64, 1169–1183 (2021). https://doi.org/10.1007/s00125-021-05384-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-021-05384-9