Abstract
Purpose
Dopamine transporters (DAT) modulate pre-synaptic dopamine and physiological functions such as movement and reward. DAT also mirrors disease state in neurological disorders, rendering it an essential diagnostic target. [18F]PR04.MZ is a new PET imaging agent for DAT with an improved affinity and selectivity profile, for which we here describe the complete pharmacokinetic evaluation in healthy controls.
Methods
Thirty-two healthy subjects underwent T1-weighted MRI and dynamic PET scans for 180 min with arterial blood sampling (n = 5) or 90 min without blood sampling (n = 25) after injection of 197.6 ± 12.2 MBq [18F]PR04.MZ. Blood and plasma metabolite analysis were performed. MRI-based normalization of brain images, delineation of VOIs, and kinetic modeling was conducted to determine distribution volumes (Vt) and binding potentials (BPnd). The impact of scan duration was evaluated and repeated PET scans were performed to assess test-retest variability (n = 5). A static imaging protocol has been validated for clinical applications.
Results
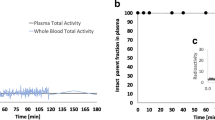
[18F]PR04.MZ showed rapid metabolization in circulation, very high uptake in striatum and midbrain, and very low non-specific binding. The two-tissue compartment model 2TCM provided best fits for measured time-activity-curves and calculated Vts in putamen, caudate, substantia nigra pars compacta (SNpc), and cerebellar cortex were 11.83, 9.73, 2.12, and 0.57, respectively. All non-invasive models correlated well with BPnd values derived from 2TCM but underestimated DAT availability by about 28–33%. Of those, simplified reference tissue model (SRTM) provided the best fits, lowest Akaike Information Criteria values, and BPnd values of 14.82, 11.95, and 2.63 in putamen, caudate, and SNpc, respectively. BPnd estimates for striatal regions and SNpc were stable between 90 and 130 min post-injection. Test-retest results were excellent, showing low variability in all and excellent reliability in most relevant regions. Static imaging from 60 to 90-min post-injection is a viable alternative for quantification.
Conclusions
[18F]PR04.MZ is a PET tracer with very high affinity, selectivity, and specific uptake in striatum and midbrain. 2TCM and SRTM provide good fits, high and stable Vts or BPnds, and good test-retest reliability for precise quantification of DAT in human subjects.





Similar content being viewed by others
References
Vaughan RA, Foster JD. Mechanisms of dopamine transporter regulation in normal and disease states. Trends Pharmacol Sci. 2013;34(9):489–96.
Bannon MJ. The dopamine transporter: role in neurotoxicity and human disease. Toxicol Appl Pharmacol. 2005;204(3):355–60.
Strafella AP, Bohnen NI, Perlmutter JS, et al. Molecular imaging to track Parkinson’s disease and atypical parkinsonisms: New imaging frontiers. Mov Disord. 2017;32(2):181–92.
Dickson DW, Braak H, Duda JE, et al. Neuropathological assessment of Parkinson’s disease: refining the diagnostic criteria. Lancet Neurol. 2009;8(12):1150–7.
Yaqub M, Boellaard R, van Berckel BN, et al. Quantification of dopamine transporter binding using [18F]FP-beta-CIT and positron emission tomography. J Cereb Blood Flow Metab. 2007;27(7):1397–406.
Sasaki T, Ito H, Kimura Y, et al. Quantification of dopamine transporter in human brain using PET with 18F-FE-PE2I. J Nucl Med. 2012;53(7):1065–73.
Nye JA, Votaw JR, Bremmer JD, et al. Quantification of dopamine transporter density with [18F]FECNT PET in healthy humans. Nucl Med Biol. 2014;41(3):217–22.
Kazumata K, Dhawan V, Chaly T, et al. Dopamine transporter imaging with fluorine-18-FPCIT and PET. J Nucl Med. 1998;39(9):1521–30.
Ariclot N, Vercouillie J, Malherbe C, et al. PET imaging of dopamine transporter with 18F-LBT999: first human exploration. J Nucl Med. 2017;58(suppl 1):276.
Lee I, Kim JS, Park JY, et al. Head-to-head comparison of 18F-FP-CIT and 123I-FP-CIT for dopamine transporter imaging in patients with Parkinson’s disease: a preliminary study. Synapse. 2018;72(7):e22032.
Jakobson Mo S, Axelsson J, Jonasson L, et al. Dopamine transporter imaging with [18F]FE-PE2I PET and [123I]FP-CIT SPECT-a clinical comparison. EJNMMI Res. 2018;8(1):100.
Riss PJ, Hummerich R, Schloss P. Synthesis and monoamine uptake inhibition of conformationally constrained 2beta-carbomethoxy-3beta-phenyl tropanes. Org Biomol Chem. 2009;7(13):2688–98.
Riss PJ, Debus F, Hummerich R, et al. Ex vivo and in vivo evaluation of [18F]PR04.MZ in rodents: a selective dopamine transporter imaging agent. ChemMedChem. 2009;4(9):1480–7.
Goodman MM, Kilts CD, Keil R, et al. 18F-labeled FECNT: a selective radioligand for PET imaging of brain dopamine transporters. Nucl Med Biol. 2000;27(1):1–12.
Chalon S, Hall H, Saba W, et al. Pharmacological characterization of (E)-N-(4-fluorobut-2-enyl)-2beta-carbomethoxy-3beta-(4′-tolyl)nortropane (LBT-999) as a highly promising fluorinated ligand for the dopamine transporter. J Pharmacol Exp Ther. 2006;317(1):147–52.
Chalon S, Garreau L, Emond P, et al. Pharmacological characterization of (E)-N-(3-iodoprop-2-enyl)-2beta-carbomethoxy-3beta-(4′-methylphenyl)n ortropane as a selective and potent inhibitor of the neuronal dopamine transporter. J Pharmacol Exp Ther. 1999;291(2):648–54.
Riss PJ, Roesch F. Efficient microwave-assisted direct radiosynthesis of [18F]PR04.MZ and [18F]LBT999: selective dopamine transporter ligands for quantitative molecular imaging by means of PET. Bioorg Med Chem. 2009;17(22):7630–4.
Riss PJ, Hooker JM, Shea C, et al. Characterisation of [11C]PR04.MZ in Papio anubis baboon: a selective high-affinity radioligand for quantitative imaging of the dopamine transporter. Bioorg Med Chem Lett. 2012;22(1):679–82.
Juri C, Chana P, Kramer V, et al. Imaging nigrostriatal dopaminergic deficit in Holmes tremor with 18F-PR04.MZ-PET/CT. Clin Nucl Med. 2015;40(9):740–1.
Kramer V, Juri C, Chana-Cuevas P, et al. Dopamine transporter quantification by PET with [18F]PR04.MZ in patients with early Parkinson’s disease. J Nucl Med. 2014;55(suppl 1):1829.
Kramer V, Pruzzo R, Chana-Cuevas P, et al. Initial human PET studies with [18F]PR04.MZ for quantification of striatal and extrastriatal dopamine transporters. J Nucl Med. 2013;54(suppl 2):416.
Hammers A, Allom R, Koepp MJ, et al. Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Hum Brain Mapp. 2003;19(4):224–47.
Lammertsma AA, Bench CJ, Hume SP, et al. Comparison of methods for analysis of clinical [11C]raclopride studies. J Cereb Blood Flow Metab. 1996;16:10.
Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. NeuroImage. 1996;4(3):153–8.
Baumgartner R, Joshi A, Feng D, Zanderigo F, Ogden RT. Statistical evaluation of test-retest studies in PET brain imaging. EJNMMI Res. 2018;8(1):13.
Darcourt J, Booij J, Tatsch K et al. EANM procedure guidelines for brain neurotransmission SPECT using (123)I-labelled dopamine transporter ligands, version 2. Eur J Nucl Med Mol Imaging. 2010;37(2):443–50.
Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15(2):155–63.
Chana-Cuevas P, Juri C, Kramer V et al. 2016 Quantification of striatal dopamine transporters with [18F]PR04.MZ in patients with progressive supranuclear palsy and Parkinson’s disease. Mov Disord.;31(suppl 2). https://www.mdsabstracts.org/abstract/quantification-of-striatal-dopamine-transporters-with-18fpr04-mz-in-patients-with-progressive-supranuclear-palsy-and-parkinsons-disease/.
Acknowledgments
We would like to thank Dr. Geoff Warnock (PMod Technologies), Irene Coudeu, and Ana Hurtado (Positronmed) for their help during PET studies; Dr. Evelyng Faure (FALP, Chile) for acquiring MRI scans and Carlos Elgueta; and Dr. Mario Avila (Positronpharma) for assistance in tracer production.
Funding
This study was in part funded by the InnovaChile (CORFO), project 13PIE-21682. Carlos Juri was funded by Conicyt-Chile, project FONDECYT 11130534.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The study was approved by the regional ethics committee board (CEC SSM Oriente, permit 20140520) and written informed consent has been obtained from all participants.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Key points
Question: To study and describe the pharmacokinetics and an adequate imaging and quantification protocol for [18F]PR04.MZ.
Pertinent findings: We evaluated metabolism, regional brain uptake, kinetic modeling, impact of scan duration, and test-retest variability and showed that [18F]PR04.MZ is a highly selective PET tracer for DAT, providing excellent imaging contrast and stable, reliable quantification outcomes within a reasonable time frame.
Implications for patient care: The high specific uptake, in particular, in SNpc is an advantage over existing methods and may have a clinical impact for patients being evaluated for movement disorders.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Translational research
Electronic supplementary material
ESM 1
(DOCX 1534 kb)
Rights and permissions
About this article
Cite this article
Kramer, V., Juri, C., Riss, P.J. et al. Pharmacokinetic evaluation of [18F]PR04.MZ for PET/CT imaging and quantification of dopamine transporters in the human brain. Eur J Nucl Med Mol Imaging 47, 1927–1937 (2020). https://doi.org/10.1007/s00259-019-04594-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-019-04594-z