Abstract
Background
The aim of this study was to investigate the role of IL-17A in the cancer microenvironment and the recurrence of triple negative breast cancer (TNBC).
Methods
Using human TNBC cell lines, the role of IL17-A was investigated by knocked down of IL-17A (ΔIL-17A) and by administration of IL-17A into the culture medium. Cell proliferation assays, migration assays, as well as Western blot analysis and real-time PCR, were used to evaluate IL-17A-related signaling. Three types of 4T1 cells were implanted into BALB/c mice, namely wild type (WT), ΔIL-17A, and WT + neutralizing IL-17 antibody (WT + Ab) cells. Tumor weight, necrosis area, and the number of circulating tumor cells (CTCs) were measured. Immunohistochemistry and Western blotting were used to analyze expression of CD34, CD8, and TGF-β1 as well as anoikis resistance. The Kaplan–Meier’s method was used to correlate IL-17A expression and patient outcome, including disease-free survival (DFS) and overall survival (OS).
Results
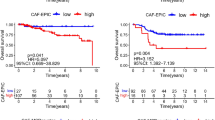
Our results demonstrated that IL-17A was able to stimulate the migratory activity, but not the growth rate, of MDA-MB-231/468 cells. In vivo, for the ΔIL-17A group, there was an increase in necrosis area, a decrease in tumor CD34 expression and a reduction in the number of CTCs. Furthermore, in WT + Ab group, there was a decreased in tumor expression of CD34, fewer CD8 ( +) cells, and fewer CTCs, but an increase in expression of TGF-β1 expression. Both of the above were compared to the WT group. Knockdown of IL-17A also decreased anoikis resistance in human TNBC and the murine 4T1 cell lines. Kaplan–Meier analysis disclosed a negative correlation between tumor expression of IL-17A and OS in TNBC patients.
Conclusion
We conclude that IL-17A promotes migratory and angiogenic activity in tumors, enhances anoikis resistance, and modulates the immune landscape of the tumor microenvironment such changes favor cancer metastasis.







Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
References
Nik-Zainal S, Davies H, Staaf J et al Landscape of somatic mutations in 560 breast cancer whole-genome sequences. Doi: D - NLM: EMS68344 EDAT- 2016/05/03 06:00 MHDA- 2016/06/29 06:00 CRDT- 2016/05/03 06:00 PHST- 2015/06/29 00:00 [received] PHST- 2016/03/17 00:00 [accepted] PHST- 2016/05/03 06:00 [entrez] PHST- 2016/05/03 06:00 [pubmed] PHST- 2016/06/29 06:00 [medline] AID - nature17676 [pii] AID - https://doi.org/10.1038/nature17676 [doi] PST - ppublish
Podo F, Buydens LMC, Degani H et al (2010) Triple-negative breast cancer: Present challenges and new perspectives. Mol Oncol 4:209–229 (Https://doi.org/10.1016/j.molonc.2010.04.006)
Sharma P (2016) Biology and management of patients with triple-negative breast cancer. Oncologist 21:1050–1062. https://doi.org/10.1634/theoncologist.2016-0067
Thike AA, Cheok PY, Jara-Lazaro AR, Tan B, Tan P, Tan PH (2010) Triple-negative breast cancer: clinicopathological characteristics and relationship with basal-like breast cancer. Modern Pathol 23:123–133. https://doi.org/10.1038/modpathol.2009.145
Lehmann BD, Pietenpol JA, Tan AR (2015) Triple-negative breast cancer: molecular subtypes and new targets for therapy. American Society of Clinical Oncology Educational Book. E31-e9. https://doi.org/10.14694/edbook_AM.2015.35.e31
Abramson VG, Lehmann BD, Ballinger TJ, Pietenpol JA (2015) Subtyping of triple-negative breast cancer: implications for therapy. Cancer 121:8–16. https://doi.org/10.1002/cncr.28914
Tseng L-M, Chiu J-H, Liu C-Y, Tsai Y-F, Wang Y-L, Yang C-W, Shyr Y-M (2017) A comparison of the molecular subtypes of triple-negative breast cancer among non-Asian and Taiwanese women. Breast Cancer Res Treat 163:241–254. https://doi.org/10.1007/s10549-017-4195-7
Widera D, Martínez Aguilar R, Cottrell GS, (2019) Toll-like receptor 4 and protease-activated receptor 2 in physiology and pathophysiology of the nervous system: more than just receptor cooperation? Neural Regen Res 14:1196–1201. https://doi.org/10.4103/1673-5374.251290
Jung MK, Kwak J-E, Shin E-C (2017) IL-17A-producing Foxp3+ regulatory T cells and human diseases. Immune Netw 17:276–286
Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA (2014) Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res 2014:149185. https://doi.org/10.1155/2014/149185
Fabre JAS, Giustinniani J, Garbar C, Merrouche Y, Antonicelli F, Bensussan A (2018) The interleukin-17 family of cytokines in breast cancer. Int J Mol Sci. https://doi.org/10.3390/ijms19123880
Welte T, Zhang XH (2015) Interleukin-17 could promote breast cancer progression at several stages of the disease. Mediators Inflamm 2015:804347. https://doi.org/10.1155/2015/804347
Oeckinghaus A, Ghosh S (2009) The NF-kappab family of transcription factors and its regulation. Cold Spring Harb Perspect Biol 1:a000034. https://doi.org/10.1101/cshperspect.a000034
Zhang Y, Lv Y, Niu Y, Su H, Feng A (2017) Role of circulating tumor cell (CTC) monitoring in evaluating prognosis of triple-negative breast cancer patients in China. Med Sci Monit 23:3071–9. https://doi.org/10.12659/msm.902637
Paoli P, Giannoni E, Chiarugi P (2013) Anoikis molecular pathways and its role in cancer progression. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 1833:3481–98. Https://doi.org/https://doi.org/10.1016/j.bbamcr.2013.06.026
Chiu J-H, Tseng L-M, Huang T-T, Liu C-Y, Wang J-Y, Huang C-P, Tsai Y-F, Hsu C-Y (2020) MEGF11 is related to tumour recurrence in triple negative breast cancer via chemokine upregulation. Sci Rep 10:8060. https://doi.org/10.1038/s41598-020-64950-0
Pulaski BA, Ostrand-Rosenberg S (2000) Mouse 4T1 Breast tumor model. Curr Protoc Immunol 39:20.2.1–20.2.16. https://doi.org/10.1002/0471142735.im2002s39
Chiu JH, Chen FP, Tsai YF, Lin MT, Tseng LM, Shyr YM (2017) Effects of Chinese medicinal herbs on expression of brain-derived Neurotrophic factor (BDNF) and its interaction with human breast cancer MDA-MB-231 cells and endothelial huvecs. BMC Complement Altern Med 17:401. https://doi.org/10.1186/s12906-017-1909-7
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Chomczynski P, Sacchi N (2006) The single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction: twenty-something years on. Nat Protoc 1:581–585. https://doi.org/10.1038/nprot.2006.83
Huang S, Houghton PJ (2003) Targeting mtor signaling for cancer therapy. Curr Opin Pharmacol 3:371–377. https://doi.org/10.1016/S1471-4892(03)00071-7
Tata A, Woolman M, Ventura M et al (2016) Rapid detection of necrosis in breast cancer with desorption electrospray ionization mass spectrometry. Sci Rep 6:35374. https://doi.org/10.1038/srep35374
Folkman J (1971) Tumor angiogenesis: therapeutic implications. New Engl J Med 285:1182–1186. https://doi.org/10.1056/NEJM197111182852108
Wang R, Lou X, Feng G et al (2019) IL-17A-stimulated endothelial fatty acid β-oxidation promotes tumor angiogenesis. Life Sci 229:46–56 (Https://doi.org/10.1016/j.lfs.2019.05.030)
Sidney LE, Branch MJ, Dunphy SE, Dua HS, Hopkinson A (2014) Concise review: evidence for CD34 as a common marker for diverse progenitors. Stem Cells 32:1380–1389. https://doi.org/10.1002/stem.1661
Sun S-C (2012) The noncanonical NF-κb pathway. Immunol Rev 246:125–140. https://doi.org/10.1111/j.1600-065X.2011.01088.x
Ouyang W, Beckett O, Ma Q, Li MO (2010) Transforming growth factor-β signaling curbs thymic negative selection promoting regulatory T cell development. Immunity 32:642–653 (Https://doi.org/10.1016/j.immuni.2010.04.012)
Ouyang W, Oh Soyoung A, Ma Q, bivonamichael R, Zhu J, liming O, (2013) TGF-β cytokine signaling promotes CD8+ T cell development and low-affinity CD4+ T cell homeostasis by regulation of interleukin-7 receptor α expression. Immunity 39:335–346 (Https://doi.org/10.1016/j.immuni.2013.07.016)
Pan MH, Chiou YS, Tsai ML, Ho CT (2011) Anti-inflammatory activity of traditional Chinese medicinal herbs. J Tradit Complement Med 1:8–24. https://doi.org/10.1016/s2225-4110(16)30052-9
Gagliani N, Vesely MCA, Iseppon A et al (2015) Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature 523:221–225. https://doi.org/10.1038/nature14452
Sharma M, Kaveri SV, Bayry J (2013) Th17 cells, pathogenic or not? TGF-β3 imposes the embargo. Cell Mol Immunol 10:101–102. https://doi.org/10.1038/cmi.2012.72
Nam JS, Terabe M, Kang MJ et al (2008) Transforming growth factor beta subverts the immune system into directly promoting tumor growth through interleukin-17. Cancer Res 68:3915–3923. https://doi.org/10.1158/0008-5472.CAN-08-0206
Gupta GP, Massagué J (2006) Cancer metastasis: building a framework. Cell 127:679–695 (Https://doi.org/10.1016/j.cell.2006.11.001)
Lou X-L, Sun J, Gong S-Q, Yu X-F, Gong R, Deng H (2015) Interaction between circulating cancer cells and platelets: clinical implication. Chin J Cancer Res 27:450–460. https://doi.org/10.3978/j.issn.1000-9604.2015.04.10
Liu Q, Liao Q, Zhao Y (2016) Myeloid-derived suppressor cells (MDSC) facilitate distant metastasis of malignancies by shielding circulating tumor cells (CTC) from immune surveillance. Med Hypotheses 87:34–39 (Https://doi.org/10.1016/j.mehy.2015.12.007)
Mazel M, Jacot W, Pantel K et al (2015) Frequent expression of PD-L1 on circulating breast cancer cells. Mol Oncol 9:1773–1782 (Https://doi.org/10.1016/j.molonc.2015.05.009)
Tseng JY, Yang CY, Liang SC et al (2014) Interleukin-17A modulates circulating tumor cells in tumor draining vein of colorectal cancers and affects metastases. Clin Cancer Res 20:2885–2897. https://doi.org/10.1158/1078-0432.CCR-13-2162
Hanahan D, Weinberg Robert A (2011) Hallmarks of Cancer: The Next Generation. Cell 144:646–674 (Https://doi.org/10.1016/j.cell.2011.02.013)
Yan L, Anderson GM, dewitte M, Nakada MT, (2006) Therapeutic potential of cytokine and chemokine antagonists in cancer therapy. Eur J Cancer 42:793–802 (Https://doi.org/10.1016/j.ejca.2006.01.013)
Merrouche Y, Fabre J, Cure H et al. (2016) IL-17E synergizes with EGF and confers in vitro resistance to EGFR-targeted therapies in TNBC cells. Oncotarget 7:53350–61. https://doi.org/10.18632/oncotarget.10804
Reis J, Vender R, Torres T (2019) Bimekizumab: the first dual inhibitor of interleukin (IL)-17A and IL-17F for the treatment of psoriatic disease and ankylosing spondylitis. BioDrugs 33:391–399. https://doi.org/10.1007/s40259-019-00361-6
Acknowledgements
We are in debt to Chou, SS, Wang, YL, and Hsu, TH for their technique supports. This work was supported by the biobank from the Division of Experimental Surgery, Department of Surgery, Taipei Veterans General Hospital. We thank the Taiwan Animal Consortium (MOST 107-2319-B-001-002)--Taiwan Mouse Clinic, which is funded by the Ministry of Science and Technology (MOST) of Taiwan, for technical support in the IVIS animal experiment. We also thanks Ralph Kirby for English edition of this manuscript. Funding was provided by Ministry of Health and Welfare (Grant No. MOHW108-TDU-B-212-112015) and Health Promotion Administration, Ministry of Health and Welfare (Grant No. MOHW109-TDU-B-212-010001).
Funding
This work was supported by grants from the Ministry of Health and Welfare (Center of Excellence for Cancer Research at Taipei Veterans General Hospital phase II, MOHW108-TDU-B-212–112,015, phase III, MOHW109-TDU-B-212–010,001).
Author information
Authors and Affiliations
Contributions
JHC formed the idea. LMT and JHC contributed equally in this manuscript. LMT supervised the experiments. CYL, LMT, YFT, CCH, and YSL provided clinical samples and data. CPH performed the experiments. CYH read the pathology slices.
Corresponding author
Ethics declarations
Conflict of interest
We declare that we have no conflicts of interest, including financial and non-financial interests such as the following items.
-
Unpaid membership in a government or non-governmental organization.
-
Unpaid membership in an advocacy or lobbying organization.
-
Unpaid advisory position in a commercial organization.
-
Writing or consulting for an educational company.
-
Acting as an expert witness.
Ethics approval and consent to participate
Study protocols involving experimental mice followed ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines and were approved by the Institutional Animal Committee of and Taipei Veterans General Hospital (No. 2018–029). The human study for tumor tissue utilization from the biobank was approved by the Institutional Review Board of Taipei Veterans General Hospital (# 2013–10-020BC).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tsai, YF., Huang, CC., Lin, YS. et al. Interleukin 17A promotes cell migration, enhances anoikis resistance, and creates a microenvironment suitable for triple negative breast cancer tumor metastasis. Cancer Immunol Immunother 70, 2339–2351 (2021). https://doi.org/10.1007/s00262-021-02867-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-021-02867-x