Abstract
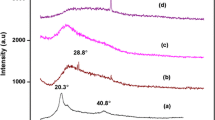
Limited global resources of lithium lead to the consideration of magnesium ion batteries as potential energy storage devices. Magnesium ion batteries have potential for high energy density but require new types of electrode and electrolytes for practical applications. Solid polymer electrolytes offer the opportunity for increased safety and broader electrochemical stability relative to traditional electrolytes. Herein, we report the development of solid polymer electrolyte for magnesium ion batteries based on triblock copolymer poly(vinylidene chloride-co-acrylonitrile-co-methyl methacrylate) (poly(VdCl-co-AN-co-MMA)). The polymer electrolytes are prepared by solution-casting technique using poly(VdCl-co-AN-co-MMA) with various concentrations (10 wt%, 20 wt%, 30 wt%, and 40 wt%) of magnesium chloride (MgCl2) salt. Among the prepared polymer electrolytes, the highest magnesium-ion-conducting polymer electrolyte is 70 wt% poly(VdCl-co-AN-co-MMA):30 wt% MgCl2 polymer–salt composition by electrochemical impedance measurements, and the obtained value of ionic conductivity is found to be in the order of 10−5 S cm−1. Addition of plasticizer succinonitrile in various concentrations (0.1 wt%, 0.2 wt%, 0.3 wt% and 0.4 wt%) with the identified polymer electrolyte of highest conductivity shows increased values of conductivity up to the order of 10−3 S cm−1. Observable changes in crystalline/amorphous nature of the polymer are analyzed using X-ray diffraction pattern. Glass transition temperature of polymer electrolytes has been found using differential scanning calorimetric studies. Transference number measurements have been made to confirm the ionic conductivity. The electrochemical stability for highest conducting plasticized polymer electrolyte is obtained from linear sweep voltammetry as 3.3 V. A primary magnesium ion battery has been constructed with prepared electrolyte of highest conductivity, and its performance and discharge characteristics are also analyzed. The open-circuit voltage of 2.18 V is obtained with the constructed primary magnesium ion battery.













Similar content being viewed by others
References
Tan Y-H, Yao W-T, Zhang T, Ma T, Lei-Lei L, Zhou F, Yao H-B, Shu-Hong Yu (2018) High voltage magnesium-ion battery enabled by nanocluster Mg3Bi2 alloy anode in noncorrosive electrolyte. ACS Nano. https://doi.org/10.1021/acsnano.8b01847
Huie MM, Bock DC, Takeuchi ES, Marschilok AC, Takeuchi KJ (2015) Cathode materials for magnesium and magnesium-ion based batteries. Coord Chem Rev 287:15–27
Zhou L, Liu Q, Zhang Z, Zhang K, Xiong F, Tan S, An Q, Kang Y-M, Zhou Z, Mai L (2018) Interlayer-spacing-regulated VOPO4 nanosheets with fast kinetics for high-capacity and durable rechargeable magnesium batteries. Adv Mater 30:1801984
Bančič T, Bitenc J, Pirnat K, Lautar AK, Grdadolnik J, Vitanova AR, Dominko R (2018) Electrochemical performance and redox mechanism of naphthalene-hydrazinediimide polymer as a cathode in magnesium battery. J Power Sour 395:25–30
Penki TR, Valurouthu G, Shivakumara S, Sethuraman VA, Munichandraiah N (2018) In-situ synthesis of bismuth (Bi)/reduced graphene oxide (RGO) nanocomposites as high capacity anode materials for Mg-ion battery. https://doi.org/10.1039/x0xx00000x
Saha P, Kanchan Datta M, Velikokhatnyi OI, Manivannan A, Alman D, Kumta PN (2014) Rechargeable magnesium battery: current status and key challenges for the future. https://doi.org/10.1016/j.pmatsci.2014.04.001
Zhao X, Zhao Z, Miao Y (2018) Chloride ion-doped polypyrrole nanocomposite as cathode material for rechargeable magnesium battery. https://doi.org/10.1016/j.materresbull.2018.01.012
Mesallam M, Sheha E, Kamar EM, Sharma N (2018) Graphene and magnesiated graphene as electrodes for magnesium ion batteries. https://doi.org/10.1016/j.matlet.2018.08.080
Nacimiento F, Cabello M, Pérez-Vicente C, Alcántara R, Lavela P, Ortiz GF, Tirado JL (2018) On the mechanism of magnesium storage in micro and nano-particulate tin battery electrodes. Nanomaterials 8:501
Zhang Z, Dong S, Cui Z, Du A, Li G, Cui G (2018) Rechargeable magnesium batteries using conversion-type cathodes: a perspective and minireview. Small Methods 1800020
Li Y, Yerian JA, Khan SA, Fedkiw PS (2006) Crosslinkable fumed silica-based nanocomposite electrolytes for rechargeable lithium batteries. J Power Sour 161:1288–1296
Hallinan DT Jr, Balsara NP (2013) Polymer electrolytes. Annu Rev Mater Res 43:503–525
Ahmad S (2009) Polymer electrolytes: characteristics and peculiarities. Ionics 15:309–321
Ibrahim S, Yassin MM, Ahmad R, Johan MR (2011) Effects of various LiPF6 salt concentrations on PEO-based solid polymer electrolytes. Ionics 17:399–405
Meyer WH (1998) Polymer electrolytes for lithium-ion batteries. Adv Mater 10(6):439
Pandey M, Joshi GM, Ghosh NN (2016) Ionic conductivity and diffusion coefficient of barium-chloride-based polymer electrolyte with poly(vinyl alcohol)-poly(4-styrenesulphonic acid) polymer complex. Bull Mater Sci 40:655–666
Khutia M, Joshi GM, Deshmukh K, Pandey M (2015) Optimization of dielectric constant of polycarbonate/polystyrene modified blend by ceramic metal oxide. Polym-Plast Technol Eng 54:383–389
Fergus JW (2010) Ceramic and polymeric solid electrolytes for lithium-ion batteries. J Power Sour 195:4554–4569
Zhang Z, Fang S (2000) Novel network polymer electrolytes based on polysiloxane with internal plasticizer. Electrochim Acta 45:2131–2138
Soo PP, Huang B, Jang Y-I, Chiang Y-M, Sadoway DR, Mayes AM (1999) Rubbery block copolymer electrolytes for solid-state rechargeable lithium batteries. J Electrochem Soc 146(1):32–37
Arges CG, Kambe Y, Dolejsi M, Wu G, Segal-Pertz T, Ren J, Cao C, Craig GSW, Nealey PF (2017) Interconnected ionic domains enhance conductivity in microphase separated block copolymer electrolytes. https://doi.org/10.1039/c6ta10838e
Devaux D, Gle D, Phan TNT, Gigmes D, Giroud E, Deschamps M, Denoyel R, Bouchet R (2015) Optimization of block copolymer electrolytes for lithium metal batteries. https://doi.org/10.1021/acs.chemmater.5b01273
Saikia D, Hao-Yiang W, Pan Y-C, Lin C-P, Huang K-P, Chen K-N, Fey George TK, Kao H-M (2011) Highly conductive and electrochemically stable plasticized blend polymer electrolytes based on PVdF-HFP and triblock copolymer PPG-PEG-PPG diamine for Li-ion batteries. J Power Sourc 196:2826–2834
Young NP, Devaux D, Khurana R, Coates GW, Balsara NP (2014) Investigating polypropylene-poly(ethylene oxide)-polypropylene triblock copolymers as solid polymer electrolytes for lithium batteries. Solid State Ionics 263:87–94
Young W-S, Epps TH (2012) Ionic conductivities of block copolymer electrolytes with various conducting pathways: sample preparation and processing considerations. Macromolecules 45:4689–4697
Bouchet R, Phan TNT, Beaudoin E, Devaux D, Davidson P, Bertin D, Denoyel R (2014) Charge transport in nanostructured PS–PEO–PS triblock copolymer electrolytes. Macromolecules 47:2659–2665
Inceoglu S, Rojas AA, Didier Devaux X, Chen C, Stone GM, Balsara NP (2014) Morphology–conductivity relationship of single-ion-conducting block copolymer electrolytes for lithium batteries. ACS Macro Lett 3:510–514
Renaud Bouchet, Sébastien Maria, Rachid Meziane, Abdelmaula Aboulaich, Livie Lienafa, Jean-Pierre Bonnet, Trang N. T. Phan, Denis Bertin, Didier Gigmes, Didier Devaux, Renaud Denoyel, Michel Armand (2013) Single-ion BAB triblock copolymers as highly efficient electrolytes for lithium-metal batteries, https://doi.org/10.1038/nmat3602
Inbavalli D, Selvasekarapandian S, Sanjeeviraja C, Baskaran R, Kawamura J, Masuda Y (2013) Structural, thermal, morphological and electrical conductivity analysis of proton conducting tri block copolymer P(VdCl-Co-AN-Co-MMA) based electrolytes. In. J Electroact Mater 1:71–78
Inbavalli D, Selvasekarapandian S, Sanjeeviraja C et al (2015) Analysis of P(VdCl-co-AN-co-MMA)-LiClO4-EC triblock copolymer electrolytes. Bull Mater Sci 38:183
Inbavalli D, Selvasekarapandian S, Sanjeeviraja C et al (2013) Analysis of lithium ion conducting P(VdCl-co-AN-co-MMA)-LiClO4-DMF tri block copolymer electrolytes. Indian J Appl Res 3(12). ISSN-2249-555X
Anbazhakan K, Selvasekarapandiyan S, Monisha S et al (2017) Lithium ion conductivity and dielectric properties of P(VdCl-co-AN-co-MMA)-LiCl-EC triblock co-polymer electrolytes. Ionics 23:2663
Pandey M, Joshi GM, Ghosh NN (2016) Electrical performance of lithium ion based polymer electrolyte with polyethylene glycol and polyvinyl alcohol network. Int J Polym Mater Polym Biomater 65:759–768
Eom H-C, Park H, Yoon H-S (2010) Preparation of anhydrous magnesium chloride from ammonium magnesium chloride hexahydrate. Adv Powder Technol 21:125–130
Zhang Z, Xuchen L, Yang S, Pan F (2012) Preparation of anhydrous magnesium chloride from magnesia. Ind Eng Chem Res 51:9713–9718
Hodge RM (1996) Water absorption and states of water in semicrystalline poly(vinyl alcohol) films. Polymer 37(8):1371–1376
Rahman MYA, Ahmad A, Lee TK, Farina Y, Dahlan HM (2012) LiClO4 salt concentration effect on the properties of PVC-modified low molecular weight LENR50-based solid polymer electrolyte. J Appl Polym Sci 124:2227–2233
Polu AR, Kumar R (2013) Ionic conductivity and discharge characteristic studies of PVA-Mg(CH3COO)2 solid polymer electrolytes. Int J Polym Mater Polym Biomater 62(2):76–80
Polu AR, Kumar R (2013) Preparation and characterization of PVA based solid polymer electrolytes for electrochemical cell applications. Chin J Polym Sci 31(4):641–648
Selvalakshmi S, Vijaya N, Selvasekarapandian S, Premalatha M (2016) Biopolymer agar-agar doped with NH4SCN as solid polymer electrolyte for electrochemical cell application. J Appl Polym Sci. https://doi.org/10.1002/APP.44702
Liu W, Lee SW, Lin D, Shi F, Wang S, Sendek AD, Cui Y (2017) Enhancing ionic conductivity in composite polymer electrolytes with well-aligned ceramic nanowires. Nat Energy 2. Article number: 17035
Boukamp BA (1986) A package for Impedance/Admittance data analysis. Solid State Ion 18 & 19:136–140
Boukamp BA (1986) A Nonlinear least squares fit procedure for analysis of immittance data of electrochemical systems. Solid State Ion 20:31–44
Osman Z, Zainol NH, Samin SM, Chong WG, Md Isa KB, Othman L, Supa’at I, Sonsudin F (2014) Electrochemical impedance spectroscopy studies of magnesium-based polymethylmethacrylate gel polymer electroytes. ElectrochimicaActa 131:148–153
Kumudu Perera MAKL, Dissanayake PWSK Bandaranayake (2004) Ionic conductivity of a gel polymer electrolyte based on Mg(ClO4)2 and polyacrylonitrile (PAN). Mater Res Bull 39:1745–1751
Mathew CM, Kesavan K, Rajendran S (2014) Structural and electrochemical analysis of PMMA based gel electrolyte membranes. Int J Electrochem 2015. Article ID 494308
Aravindan V, Vickraman P (2007) A novel gel electrolyte with lithium difluoro(oxalato)borate salt and Sb2O3 nanoparticles for lithium ion batteries. Solid State Sci 9:1069–1073
Cowie JMG, Spence GH (1998) Ion conduction in macroporous polyethylene film doped with electrolytes. Solid State Ion 109:139–144
Tsunemi K, Ohno H, Tsuchida E (1983) A mechanism of ionic conduction of poly(vinylidene fluoride) lithium perchlorate hybrid films. Electrochimica 28(6):833–837
Muchakayala R, Song S, Gao S, Wang X, Fan Y (2017) Structure and ion transport in an ethylene carbonate-modified biodegradable gel polymer electrolyte. Polym Test 58:116–125
Evans J, Vincent CA, Bruce PG (1987) Electrochemical measurement of transference numbers in polymer electrolytes. Polymer 28:2324–2328
Manjuladevi R, Thamilselvan M, Selvasekarapandian S, Mangalam R, Premalatha M, Monisha S (2017) Mg-ion conducting blend polymer electrolyte based on poly(vinyl alcohol)-poly(acrylonitrile) with magnesium perchlorate. Solid State Ion 308:90–100
ShanmugaPriya S, Karthika M, Selvasekarapandian S, Manjuladevi R (2018) Preparation and characterization of polymer electrolyte based on biopolymer I-Carrageenan with magnesium nitrate. Solid State Ion 327:136–149
Mokhtar M, Majlan EH, Ahmad A, Tasirin SM, Daud WRW (2018) Effect of ZnO filler on PVA-alkaline solid polymer electrolyte for aluminum-air battery applications. J Electrochem Soc 165(11):A2483–A2492
Hallinan DT, Rausch A Jr, McGill B (2016) An electrochemical approach to measuring oxidative stability of solid polymer electrolytes for lithium batteries. Chem Eng Sci 154:34–41
Zhang Y, Zhao Y, Gosselink D, Chen P (2014) Synthesis of poly(ethylene-oxide)/nanoclay solid polymer electrolyte for all solid-state lithium/sulfur battery. Ionics. https://doi.org/10.1007/s11581-014-1176-2
Manjuladevi R, Selvasekarapandian S, Thamilselvan M, Mangalam R, Monisha, Christopher Selvin P (2018) A study on blend polymer electrolyte based on poly(vinyl alcohol)-poly(acrylonitrile) with magnesium nitrate for magnesium battery. Ionics. https://doi.org/10.1007/s11581-018-2500-z
Chung S-H, Manthiram A (2017) Lithium–sulfur batteries with the lowest self-discharge and the longest shelf life. ACS Energy Lett 2:1056–1061
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ponraj, T., Ramalingam, A., Selvasekarapandian, S. et al. Plasticized solid polymer electrolyte based on triblock copolymer poly(vinylidene chloride-co-acrylonitrile-co-methyl methacrylate) for magnesium ion batteries. Polym. Bull. 78, 35–57 (2021). https://doi.org/10.1007/s00289-019-03091-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-019-03091-5