Abstract
Purpose
In the past two decades, new biomarkers for prostate cancer detection and risk prediction have become available for clinical use. While tissue-based gene expression assays offer molecular risk assessment after diagnoses, several serum- and urine-based ‘liquid’ biomarkers are available for the pre-biopsy setting which may also play a role for active surveillance (AS).
Methods
The medical literature was queried utilizing PubMed (pubmed.ncbi.nlm.nih.gov) for all relevant original publications describing prostate cancer biomarkers that can be identified in the blood, urine, or semen. Referenced studies must have defined patient inclusion criteria and descriptions of the biomarkers. Included studies investigated the utility of liquid biomarkers for selection or monitoring of men with prostate cancer for active surveillance.
Results
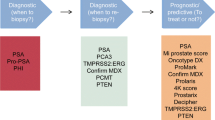
PSA is the most common and readily available biomarker for prostate cancer diagnosis and treatment. Contemporary AS guidelines consider diagnostic PSA level in addition to other clinical factors when selecting men for this approach, with most recommending that initial PSA should be under 10 ng/ml. Serum PSA changes are associated with outcomes on AS but are not adequately sensitive so drive men to secondary treatment in isolation. PSA derivates including the Prostate Health Index (phi) and the 4K Score can predict higher grade cancer and may help tailor repeat prostate biopsy strategies, but further data are needed prior to routine clinic use. Several urine-based biomarkers including PCA3 and TMPRSS2:ERG levels have also been studied in the AS setting.
Conclusions
Multiple serum- and urine-based liquid biomarkers are available for use in men with prostate cancer. For AS, serum PSA is utilized in part for patient selection as well as to monitor disease over time. Models that incorporate PSA kinetics with other clinical characteristics may help tailor surveillance strategies to reduce disease burden and health care costs over time. Several novel liquid biomarkers demonstrate promise and may eventually have applications for prostate cancer surveillance as well.
Similar content being viewed by others
References
Sanda MG, Cadeddu JA, Kirkby E et al (2018) Clinically localized prostate cancer: AUA/ASTRO/SUO guideline. Part I: risk stratification, shared decision making, and care options. J Urol 199:683
Mottet N, Bellmunt J, Bolla M et al (2017) EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. EurUrol 71:618
Auffenberg GB, Lane BR, Linsell S et al (2017) Practice- vs. physician-level variation in use of active surveillance for men with low-risk prostate cancer: implications for collaborative quality improvement. JAMA Surg 152:978
Luckenbaugh AN, Auffenberg GB, Hawken SR et al (2017) Variation in guideline concordant active surveillance followup in diverse urology practices. J Urol 197:621
Sayyid RK, Dingar D, Fleshner K et al (2017) What false-negative rates of non-invasive testing are active surveillance patients and uro-oncologists willing to accept in order to avoid prostate biopsy? Can UrolAssoc J 11:118
Stamey TA, Yang N, Hay AR et al (1987) Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N Engl J Med 317:909
Sanda MG, Cadeddu JA, Kirkby E et al (2018) Clinically localized prostate cancer: AUA/ASTRO/SUO guideline. Part II: recommended approaches and details of specific care options. J Urol 199:990
van den Bergh RC, Vasarainen H, van der Poel HG et al (2010) Short-term outcomes of the prospective multicentre “prostate cancer research international: active surveillance” study. BJU Int 105:956
Epstein JI, Walsh PC, Carmichael M et al (1994) Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA 271:368
Hamdy FC, Donovan JL, Lane JA et al (2016) 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med 375:1415
Klotz L (2005) Active surveillance with selective delayed intervention using PSA doubling time for good risk prostate cancer. EurUrol 47:16
Klotz L, Vesprini D, Sethukavalan P et al (2015) Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J ClinOncol 33:272
Bokhorst LP, Valdagni R, Rannikko A et al (2016) A decade of active surveillance in the PRIAS study: an update and evaluation of the criteria used to recommend a switch to active treatment. EurUrol 70:954
Newcomb LF, Thompson IM Jr, Boyer HD et al (2016) Outcomes of active surveillance for clinically localized prostate cancer in the prospective. Multi-Instit Canary PASS Cohort J Urol 195:313
Cooperberg MR, Brooks JD, Faino AV et al (2018) Refined analysis of prostate-specific antigen kinetics to predict prostate cancer active surveillance outcomes. EurUrol 74:211
Tosoian JJ, Loeb S, Feng Z et al (2012) Association of [-2]proPSA with biopsy reclassification during active surveillance for prostate cancer. J Urol 188:1131
Carlsson S, Maschino A, Schroder F et al (2013) Predictive value of four kallikrein markers for pathologically insignificant compared with aggressive prostate cancer in radical prostatectomy specimens: results from the European randomized study of screening for prostate cancer section Rotterdam. EurUrol 64:693
Darst BF, Chou A, Wan P et al (2020) The four-kallikrein panel is effective in identifying aggressive prostate cancer in a multiethnic population. Cancer Epidemiol Biomarkers Prev 29:1381
Lin DW, Newcomb LF, Brown MD et al (2017) Evaluating the Four Kallikrein Panel of the 4Kscore for Prediction of High-grade Prostate Cancer in Men in the Canary Prostate Active Surveillance Study. EurUrol 72:448
Olsson H, Nordstrom T, Jaderling F et al. (2020) Incorporating MRI and biomarkers in active surveillance protocols: results from the prospective Stockholm3 Active Surveillance trial (STHLM3AS). J Natl Cancer Inst. https://doi.org/10.1093/jnci/djaa131
Strom P, Nordstrom T, Aly M et al (2018) The stockholm-3 model for prostate cancer detection: algorithm update, biomarker contribution, and reflex test potential. EurUrol 74:204
Bussemakers MJ, van Bokhoven A, Verhaegh GW et al (1999) DD3: a new prostate-specific gene, highly overexpressed in prostate cancer. Cancer Res 59:5975
Nakanishi H, Groskopf J, Fritsche HA et al (2008) PCA3 molecular urine assay correlates with prostate cancer tumor volume: implication in selecting candidates for active surveillance. J Urol 179:1804
Tosoian JJ, Patel HD, Mamawala M et al (2017) Longitudinal assessment of urinary PCA3 for predicting prostate cancer grade reclassification in favorable-risk men during active surveillance. Prostate Cancer Prostatic Dis 20:339
Lin DW, Newcomb LF, Brown EC et al (2013) Urinary TMPRSS2: ERG and PCA3 in an active surveillance cohort: results from a baseline analysis in the canary prostate active surveillance study. Clin Cancer Res 19:2442
Newcomb LF, Zheng Y, Faino AV et al (2019) Performance of PCA3 and TMPRSS2:ERG urinary biomarkers in prediction of biopsy outcome in the canary prostate active surveillance study (PASS). Prostate Cancer Prostatic Dis 22:438
Das PM, Singal R (2004) DNA methylation and cancer. J ClinOncol 22:4632
Zhao F, Olkhov-Mitsel E, van der Kwast T et al (2017) Urinary DNA methylation biomarkers for noninvasive prediction of aggressive disease in patients with prostate cancer on active surveillance. J Urol 197:335
Zhao F, Vesprini D, Liu RSC et al (2019) Combining urinary DNA methylation and cell-free microRNA biomarkers for improved monitoring of prostate cancer patients on active surveillance. UrolOncol 37(297):e9
McKiernan J, Donovan MJ, O’Neill V et al (2016) A novel urine exosome gene expression assay to predict high-grade prostate cancer at initial biopsy. JAMA Oncol 2:882
Wang WW, Sorokin I, Aleksic I et al (2020) Expression of small noncoding RNAs in urinary exosomes classifies prostate cancer into indolent and aggressive disease. J Urol 204:466
Haese A, Trooskens G, Steyaert S et al (2019) Multicenter optimization and validation of a 2-Gene mRNA urine test for detection of clinically significant prostate cancer before initial prostate biopsy. J Urol 202:256
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dall’Era, M. Liquid biomarkers in active surveillance. World J Urol 40, 21–26 (2022). https://doi.org/10.1007/s00345-021-03609-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-021-03609-5