Abstract
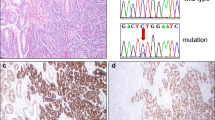
Sex cord tumor with annular tubules (SCTAT) is a highly rare type of ovarian sex cord-stromal tumor (SCST), the diagnosis of which remains to be challenging. The aim of this study was to scrutinize the utility of three immunohistochemical markers including Forkhead box protein 2 (FOXL2), SOX9, and β-catenin and DICER1 mutation status in distinguishing SCTATs from other ovarian SCSTs. Nine cases of SCTAT, 10 Sertoli-Leydig cell tumor (SCLT), 10 adult-type granulosa cell tumor (AGCT), and 8 juvenile-type granulosa cell tumor (JGCT) were included in the study. SCTATs were characterized by diffuse and strong expression of SOX9, focal and weak expression of FOXL2, and the absence of DICER1 mutation. However, AGCTs and JGCTs displayed strong and diffuse expression of FOXL2, focal/no immunoreaction for SOX9. SLCTs generally showed moderate intensity of FOXL2 and SOX9 expression. Nuclear β-catenin expression was observed in none of SLCT, 1/9 of SCTAT, 6/8 JGCT, and 4/10 AGCT cases, respectively. DICER1 hotspot mutation was detected in only 3 cases of SLCT and 2 cases of JGCT. We conclude that in addition to strong and diffuse SOX9 expression, weak/absent expression of FOXL2 is suggestive for the diagnosis of SCTAT. Hence, we suggest that inclusion of these two markers, SOX-9 and FOXL2, to the immunohistochemical panel helps in differentiation of SCTAT from other SCSTs in addition to morphologic findings. We also conclude that SCTATs of the ovary do not harbor DICER1 hotspot mutation.



Similar content being viewed by others
Data availability
Yes.
Code availability
Not applicable.
References
Young RH (2018) Ovarian sex cord-stromal tumors: reflections on a 40-year experience with a fascinating group of tumors, including comments on the seminal observations of Robert E. Scully, MD. Arch Pathol Lab Med 142(12):1459–1484
Young RH (2018) Ovarian sex cord-stromal tumours and their mimics. Pathology 50(1):11
Lim D, Oliva E (2018) Ovarian sex cord-stromal tumours: an update in recent molecular advances. Pathology 50(2):178–189
Hanley KZ, Mosunjac MB (2019) Practical review of ovarian sex cord-stromal tumors. Surg Pathol Clin 12(2):587–620
Han Y, Li S, Wu L, Zhang X, Cao D (2016) Non-Peutz-Jeghers syndrome-associated ovarian sex cord tumor with annular tubules: report of a malignant case. J Obstet Gynaecol Res 42(2):224–227
Jaegle WT, Keyser EA, Messersmith L, Brady RO, Miller C (2018) Extraovarian sex cord tumor with annular tubules discovered arising from a leiomyoma. Gynecol Oncol Rep 26:17–20
Kim J, Field A, Schultz KAP, Hill DA, Stewart DR (2017) The prevalence of DICER1 pathogenic variation in population databases. Int J Cancer 141(10):2030–2036
Goulvent T, Ray-Coquard I, Borel S, Haddad V, Devouassoux-Shisheboran M, Vacher-Lavenu MC, Pujade-Laurraine E, Savina A, Maillet D, Gillet G, Treilleux I, Rimokh R (2016) DICER1 and FOXL2 mutations in ovarian sex cord-stromal tumours: a GINECO Group study. Histopathology 68(2):279–285
Rosario R, Cohen PA, Shelling AN (2014) The role of FOXL2 in the pathogenesis of adult ovarian granulosa cell tumours. Gynecol Oncol 133(2):382–387
Leung DTH, Fuller P, Chu S (2016) Impact of FOXL2 mutations on signaling in ovarian granulosa cell tumors. Int J Biochem Cell Biol 72:4
Al-Agha OM, Huwait H, Chow C, Yang W, Senz J, Kalloger SE, Huntsman DG, Young RH, Gilks CB (2011) FOXL2 is a sensitive and specific marker for sex cord-stromal tumors of the ovary. Am J Surg Pathol 35:11
Bremmer F, Behnes CL, Schildhaus HU, Gaisa NT, Reis H, Jarry H, Radzun HJ, Stroebel P, Schweyer S (2017) The role of beta-catenin mutation and SOX9 expression in sex cord-stromal tumours of the testis. Virchows Arch 470(4):421–428
Nicol B, Yao HH (2015) Gonadal identity in the absence of pro-testis factor SOX9 and pro-ovary factor beta-catenin in mice. Biol Reprod 93(2):35
Kato N, Fukase M, Motoyama T (2004) Expression of a transcription factor, SOX9, in Sertoli-stromal cell tumors of the ovary. Int J Gynecol Pathol 23(2):180–181
Barrionuevo FJ, Hurtado A, Kim GJ, Real FM, Bakkali M, Kopp JL, Sander M, Scherer G, Burgos M, Jiménez R (2016) Sox9 and Sox8 protect the adult testis from male-to-female genetic reprogramming and complete degeneration. Elife 5:e15635
Kanai Y, Hiramatsu R, Matoba S, Kidokoro T (2005) From SRY to SOX9: mammalian testis differentiation. J Biochem 138(1):13–19
Hersmus R, Kalfa N, de Leeuw B, Stoop H, Oosterhuis JW, de Krijger R, Wolffenbuttel KP, Drop SLS, Veitia RA, Fellous M, Jaubert F, Looijenga LHJ (2008) FOXL2 and SOX9 as parameters of female and male gonadal differentiation in patients with various forms of disorders of sex development (DSD). J Pathol 215(1):31–38
Buell-Gutbrod R, Ivanovic M, Montag A, Lengyel E, Fadare O, Gwin K (2011) FOXL2 and SOX9 distinguish the lineage of the sex cord-stromal cells in gonadoblastomas. Pediatr Dev Pathol 14(5):391–395
Zhao C, Bratthauer G, Barner R, Vang R (2006) Immunohistochemical analysis of SOX9 in ovarian sertoli cell tumors and other tumors in the differential diagnosis. Int J Gynecol Pathol 26:9
Shang S, Hua F, Hu ZW (2017) The regulation of beta-catenin activity and function in cancer: therapeutic opportunities. Oncotarget 8(20):33972–33989
Chang H, Gao F, Guillou F, Taketo MM, Huff V, Behringer RR (2008) Wt1 negatively regulates beta-catenin signaling during testis development. Development 135(10):1875–1885
Kommoss S, Anglesio MS, Mackenzie R, Yang W, Senz J, Ho J, Bell L, Lee S, Lorette J, Huntsman DG, Blake Gilks C (2013) FOXL2 molecular testing in ovarian neoplasms: diagnostic approach and procedural guidelines. Mod Pathol 26(6):860–867
Kao CS, Cornejo KM, Ulbright TM, Young RH (2015) Juvenile granulosa cell tumors of the testis: a clinicopathologic study of 70 cases with emphasis on its wide morphologic spectrum. Am J Surg Pathol 39(9):1159–1169
Papanastasopoulos P, Repanti M, Damaskou V, Bravou V, Papadaki H (2008) Investigating differentiation mechanisms of the constituent cells of sex cord-stromal tumours of the ovary. Virchows Arch 453(5):465–471
Stavrinou S, Clark A, Irving J, Lee CH, Oliva E, Young R, Sriraksa R, Magdy N, van Noorden S, McCluggage WG, el-Bahrawy M (2016) Differential expression of E-cadherin and catenins in ovarian sex cord stromal tumours. Histopathology 69(2):298–306
Stewart CJ et al (2013) beta-Catenin and E-cadherin expression in stage I adult-type granulosa cell tumour of the ovary: correlation with tumour morphology and clinical outcome. Histopathology 62(2):257–266
Gullo I, Batista R, Rodrigues-Pereira P, Soares P, Barroca H, do Bom-Sucesso M, Sobrinho-Simões M (2018) Multinodular goiter progression toward malignancy in a case of DICER1 syndrome: histologic and molecular alterations. Am J Clin Pathol 149(5):379–386
Heravi-Moussavi A, Anglesio MS, Cheng SWG, Senz J, Yang W, Prentice L, Fejes AP, Chow C, Tone A, Kalloger SE, Hamel N, Roth A, Ha G, Wan ANC, Maines-Bandiera S, Salamanca C, Pasini B, Clarke BA, Lee AF, Lee CH, Zhao C, Young RH, Aparicio SA, Sorensen PHB, Woo MMM, Boyd N, Jones SJM, Hirst M, Marra MA, Gilks B, Shah SP, Foulkes WD, Morin GB, Huntsman DG (2012) Recurrent somatic DICER1 mutations in nonepithelial ovarian cancers. N Engl J Med 366(3):234–242
Kato N, Kusumi T, Kamataki A, Tsunoda R, Fukase M, Kurose A (2017) DICER1 hotspot mutations in ovarian Sertoli-Leydig cell tumors: a potential association with androgenic effects. Hum Pathol 59:41–47
Author information
Authors and Affiliations
Contributions
SO: Designed the study, performed research, collected and analyzed data, and wrote the paper.
OH: Performed research, collected and analyzed data, and wrote the paper.
AB: Performed research and collected data.
IY: Performed research and collected data.
HS: Performed research and collected data.
EY: Designed the study, performed research, collected and analyzed data, and wrote the paper.
Corresponding author
Ethics declarations
Ethics approval
Permission was obtained from the Academic Committee at Istanbul Faculty of Medicine and the study was performed in compliance with ethical standards.
Consent to participate
Not applicable.
Consent for publication
Yes.
Conflict of interest
The authors declare no conflicts of interest.
Additional information
To be used for non-life science journals: Not applicable.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Onder, S., Hurdogan, O., Bayram, A. et al. The role of FOXL2, SOX9, and β-catenin expression and DICER1 mutation in differentiating sex cord tumor with annular tubules from other sex cord tumors of the ovary. Virchows Arch 479, 317–324 (2021). https://doi.org/10.1007/s00428-021-03052-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-021-03052-2