Abstract
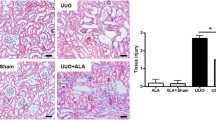
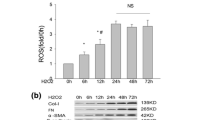
In kidney injury the accumulation of extracellular matrix (ECM) plays an important role and precedes the development of glomerulosclerosis (GS). There is great interest in agents that may interfere with such accumulation of ECM. Therefore, a rat model of GS was established to investigate the effect of all-trans retinoic acid (ATRA) on the renal expressions of matrix metalloproteinase-2 (MMP-2), matrix metalloproteinase-9 (MMP-9) and tissue inhibitor of metalloproteinase-1 (TIMP-1). Eighty Wistar rats were randomly divided into four groups: sham operation group (SHO), GS model group without treatment (GS), GS model group treated with benazepril (GB) and GS model group treated with ATRA (GA), n = 20, respectively. The disease was established in the GS rats by uninephrectomy and adriamycin (5 mg/kg) injection through the tail vein. Serum creatinine (Scr), blood urea nitrogen (BUN) and urine protein (Upro) were measured. Renal abnormality was evaluated at the end of 12 weeks. Immunohistochemical analysis was performed on renal tissue to detect the expression of collagen IV (Col-IV), fibronectin (FN), MMP-2, MMP-9 and TIMP-1 protein. MMP-2 and MMP-9 activity was detected by gelatin zymography. Real-time reverse transcription polymerase chain reaction (real-time RT-PCR) was used to detect the expression of MMP-2, MMP-9, and TIMP-1 mRNA. In comparison with group GS, group GA and group GB exhibited levels of BUN and 24 h urinary protein and a glomerulosclerosis index (GSI) that were significantly reduced (P < 0.05); the level of Scr in group GA was reduced too (P < 0.05). ATRA and benazepril also significantly down-regulated Col-IV, FN expression and TIMP-1 expression (protein and mRNA) (P < 0.05). In contrast, the expressions of MMP-2, MMP-9 mRNA and protein, and activity in groups GA and GB were enhanced (P < 0.05). However, there were no significant differences in MMP-2, MMP-9 mRNA and protein expression, or activity, between the ATRA and GB groups (P > 0.05). In conclusion, ATRA may protect renal function and step down the progression of GS by reducing the expression of TIMP-1, enhancing the expression and activity of MMP-2 and MMP-9, and regulating the ratio of MMPs/TIMPs to dynamic balance, so as to reduce the accumulation of ECM.








Similar content being viewed by others
Abbreviations
- ECM:
-
extracellular matrix
- GS:
-
glomerulosclerosis
- ATRA:
-
all-trans retinoic acid
- MMP-2:
-
matrix metalloproteinase-2
- MMP-9:
-
matrix metalloproteinase-9
- TIMP-1:
-
tissue inhibitor of metalloproteinase-1
- SHO:
-
sham operation group
- GS:
-
GS model group without treatment
- GB:
-
GS model group treated with benazepril
- GA:
-
GS model group treated with ATRA
- Scr:
-
serum creatinine
- BUN:
-
blood urea nitrogen
- Upro:
-
urine protein
- Col-IV:
-
collagen IV
- FN:
-
fibronectin
- Real-time RT-PCR:
-
real-time reverse transcription polymerase chain reaction
- MMPs:
-
matrix metalloproteinases
- TIMPs:
-
tissue inhibitor of metalloproteinases
- ADR:
-
adriamycin
- GSI:
-
glomerulosclerosis index
- H&E:
-
hematoxylin and eosin
- BCA:
-
bicinchoninic acid
- SDS:
-
sodium dodecylsulfate
- Ct:
-
threshold cycle
References
Oseto S, Moriyama T, Kawada N, Nagatoya K, Takeji M, Ando A, Yamamoto T, Imai E, Hori M (2003) Therapeutic effect of all-trans retinoic acid on rats with anti-GBM antibody glomerulonephritis. Kidney Int 64:1241–1252
Rysz J, Pedzik A, Markuszewski L, Kujawski K, Błaszczak R, Olszewski R (2005) The role of metalloproteinases in kidney pathophysiology. Pol Merkur Lekarski 19:812–815
Cheng S, Pollock AS, Mahimkar R, Olson JL, Lovett DH (2006) Matrix metalloproteinase 2 and basement membrane integrity: a unifying mechanism for progressive renal injury. FASEB J 20:1898–1900
Ahmed AK, Haylor JL, El Nahas AM, Johnson TS (2007) Localization of matrix metalloproteinases and their inhibitors in experimental progressive kidney scarring. Kidney Int 71:755–763
Liu S, Li Y, Zhao H, Chen D, Huang Q, Wang S, Zou W, Zhang Y, Li X, Huang H (2006) Increase in extracellular cross-linking by tissue transglutaminase and reduction in expression of MMP-9 contribute differentially to focal segmental glomerulosclerosis in rats. Mol Cell Biochem 284:9–17
Boyle JO (2001) Retinoid mechanisms and cyclins. Curr Oncol Rep 3:301–305
Raij L, Azar S, Keane W (1984) Mesangial immune injury, hypertension, and progressive glomerular damage in Dahl rats. Kidney Int 26:137–143
Sledge GW Jr, Qulali M, Goulet R, Bone EA, Fife R (1995) Effect of matrix metalloproteinase inhibitor batimastat on breast cancer regrowth and metastasis in athymic mice. J Natl Cancer Inst 87:1546–1551
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25:402–408
Wagner J, Dechow C, Morath C, Lehrke I, Amann K, Waldherr R, Floege J, Ritz E (2000) Retinoic acid reduces glomerular injury in a rat model of glomerular damage. J Am Soc Nephrol 11:1479–1487
Moreno-Manzano V, Manpaso F, Alique M (2003) Retinoids as a potential treatment for experimental puromycin-induced nephrosis. Br J Pharmacol 139:c828–c831
Massova I, Kotra LP, Fridman R, Mobashery S (1998) Matrix metalloproteinases: structures, evolution, and diversification. FASEB J 12:1075–1095
Fu XY, Park WC, Heineche JW (2007) Activation and silencing of matrix metalloproteinases. Semin Cell Dev Biol 6:1–12
Suzuki D, Miyazaki M, Jinde K, Koji T, Yagame M, Endoh M, Nomoto Y, Sakai H (1997) In situ hybridization studies of matrix metalloproteinase-3, tissue inhibitor of metalloproteinase-1 and type IV collagen in diabetic nephropathy. Kidney Int 52:111–119
Steinmann-Niggli K, Ziswiler R, Küng M, Marti HP (1998) Inhibition of matrix metalloproteinases attenuates anti-Thyl.1 nephritis. J Am Soc Nephrol 9:397–407
McMillan JI, Riordan JW, Couser WG, Pollock AS, Lovett DH (1996) Characterization of glomerular epithelial cell metalloproteinase as matrix metalloproteinase-9 with chanced expression in a model of membranous nephropathy. J Clin Invest 97:1094–1101
Kim ES, Sohn YW, Moon A (2007) TGF-beta-induced transcriptional activation of MMP-2 is mediated by activating transcription factor (ATF)2 in human breast epithelial cells. Cancer Lett 252:147–156
Hilgers KF, Dötsch J, Rascher W, Mann JF (2004) Treatment strategies in patients with chronic renal disease: ACE inhibitors, angiotensin receptor antagonists, or both? Pediatr Nephrol 19:956–961
Mangrum AJ, Bakris GL (2004) Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in chronic renal disease: safety issues. Semin Nephrol 24:168–175
Sun SZ, Wang Y, Li Q, Tian YJ, Liu MH, Yu YH (2006) Effects of benazepril on renal function and kidney expression of matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-2 in diabetic rats. Chin Med J (Engl) 119:814–821
Xu Q, Lucio-Cazana J, Kitamura M, Ruan X, Fine LG, Norman JT (2004) Retinoids in nephrology: promises and pitfalls. Kidney Int 66:2119–2131
Yang T, Michele DE, Park J, Smart AM, Lin Z, Brosius FC 3rd, Schnermann JB, Briggs JP (1999) Expression of peroxisomal proliferator-activated receptors and retinoid X receptors in the kidney. Am J Physiol 277:F966–F973
Schaier M, Liebler S, Schade K, Shimizu F, Kawachi H, Grone HJ, Chandraratna R, Ritz E, Wagner J (2004) Retinoic acid receptor alpha and retinoid X receptor specific agonists reduce renal injury in established chronic glomerulonephritis of the rat. J Mol Med 82:116–125
Ogawa K, Chen F, Kuang C, Chen Y (2004) Suppression of matrix metalloproteinase-9 transcription by transforming growth factor-beta is mediated by a nuclear factor-kappaB site. Biochem J 381:413–422
Darmanin S, Chen J, Zhao S, Cui H, Shirkoohi R, Kubo N, Kuge Y, Tamaki N, Nakagawa K, Hamada J, Moriuchi T, Kobayashi M (2007) All-trans retinoic acid enhances murine dendritic cell migration to draining lymph nodes via the balance of matrix metalloproteinases and their inhibitors. J Immunol 179:4616–4625
Marín MP, Esteban-Pretel G, Alonso R, Sado Y, Barber T, Renau-Piqueras J, Timoneda J (2005) Vitamin A deficiency alters the structure and collagen IV composition of rat renal basement membranes. J Nutr 135:695–701
Frankenberger M, Hauck RW, Frankenberger B, Häussinger K, Maier KL, Heyder J, Ziegler-Heitbrock HW (2001) All-trans retinoic acid selectively down-regulates matrix metalloproteinase-9 (mmp-9) and up-regulates tissue inhibitor of metalloproteinase-1 (timp-1) in human bronchoalveolar lavage cells. Mol Med 7:263–270
Manzano VM, Muñoz JC, Jiménez JR, Puyol MR, Puyol DR, Kitamura M, Cazaña FJ (2000) Human renal mesangial cells are a target for the anti-inflammatory action of 9-cis retinoic acid. Br J Pharmacol 131:1673–1683
Zaragozá R, Gimeno A, Miralles VJ, García-Trevijano ER, Carmena R, García C, Mata M, Puertes IR, Torres L, Viña JR (2007) Retinoids induce MMP-9 expression through RARalpha during mammary gland remodeling. Am J Physiol Endocrinol Metab 292:E1140–E1148
Acknowledgments
This study was supported by the Natural Science Foundation of the Guangxi Zhuang Autonomous Region (no. 0640103).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qin, YH., Lei, FY., Hu, P. et al. Effect of all-trans retinoic acid on renal expressions of matrix metalloproteinase-2, matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in rats with glomerulosclerosis. Pediatr Nephrol 24, 1477–1486 (2009). https://doi.org/10.1007/s00467-009-1166-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-009-1166-1