Abstract
Purpose
Thyroid cancer is a common subsequent malignant neoplasm in childhood cancer survivors (CCS). Patients who received radiotherapy (RT) to the head, neck, upper thorax, or total body irradiation (TBI) are considered to be at risk for subsequent thyroid cancer. Current Children’s Oncology Group screening guidelines recommend annual neck palpation. Our objective was to determine if ultrasound (US) is more sensitive and specific than palpation to detect thyroid cancer in high-risk CCS and bone marrow transplant (BMT) survivors.
Methods
Electronic medical records of patients followed in a longitudinal survivorship clinic from January 1, 2010 to December 31, 2017 were reviewed. Inclusion criteria included history of RT to the head, neck, upper thorax, or TBI for primary therapy or preparation for BMT prior to the age of 20 years.
Results
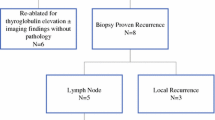
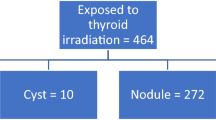
Two hundred and twenty-five patients had documented palpation and 144 (64%) also had US evaluation. Mean radiation dose was 28.6 Gy. Sixteen of 225 patients (7.1%) developed a subsequent thyroid cancer at a mean of 9.7 years from the completion of RT. Sensitivity of US was 100% compared with 12.5% for palpation. US demonstrated higher accuracy, with a receiver operating characteristic (ROC) area under the curve (AUC) of 0.87 versus 0.56 for palpation (P < 0.0001).
Conclusion
Routine screening with US was more sensitive than palpation for detection of subsequent thyroid cancer after high-risk RT in CCS and BMT survivors. Screening US may lead to earlier detection of thyroid cancer in this population. Earlier diagnosis has the potential to decrease operative complexity, and earlier definitive therapy reduces the likelihood of metastatic disease.


Similar content being viewed by others
Data availability
The authors have full control of all primary data and agree to allow this journal to review data if requested.
References
Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds) (2019) SEER cancer statistics review, 1975-2016. National Cancer Institute, Bethesda, MD, https://seer.cancer.gov/csr/1975_2016/, based on November 2018 SEER data submission, posted to the SEER web site
Wingard JR, Majhail NS, Brazauskas R, Wang Z, Sobocinski KA, Jacobsohn D, Sorror ML, Horowitz MM, Bolwell B, Rizzo JD, Socié G (2011) Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol 29(16):2230–2239
Friedman DL, Whitton J, Leisenring W, Mertens AC, Hammond S, Stovall M, Donaldson SS, Meadows AT, Robison LL, Neglia JP (2010) Subsequent neoplasms in 5-year survivors of childhood cancer: the childhood cancer survivor study. J Natl Cancer Inst 102(14):1083–1095
Turcotte LM, Neglia JP, Reulen RC, Ronckers CM, van Leeuwen F, Morton LM, Hodgson DC, Yasui Y, Oeffinger KC, Henderson TO (2018) Risk, risk factors, and surveillance of subsequent malignant neoplasms in survivors of childhood cancer: a review. J Clin Oncol 36(21):2145–2152
Turcotte LM, Liu Q, Yasui Y, Arnold MA, Hammond S, Howell RM, Smith SA, Weathers RE, Henderson TO, Gibson TM, Leisenring W, Armstrong GT, Robison LL, Neglia JP (2017) Temporal trends in treatment and subsequent neoplasm risk among 5-year survivors of childhood cancer, 1970-2015. JAMA 317(8):814–824
Inskip PD, Sigurdson AJ, Veiga L et al (2016) Radiation-related new primary solid cancers in the childhood cancer survivor study: comparative radiation dose response and modification of treatment effects. Int J Radiat Oncol Biol Phys 94(4):800–807
Veiga LHS, Holmberg E, Anderson H, Pottern L, Sadetzki S, Adams MJ, Sakata R, Schneider AB, Inskip P, Bhatti P, Johansson R, Neta G, Shore R, de Vathaire F, Damber L, Kleinerman R, Hawkins MM, Tucker M, Lundell M, Lubin JH (2016) Thyroid cancer after childhood exposure to external radiation: an updated pooled analysis of 12 studies. Radiat Res 185(5):473–484
Kovalchik SA, Ronckers CM, Veiga LHS, Sigurdson AJ, Inskip PD, de Vathaire F, Sklar CA, Donaldson SS, Anderson H, Bhatti P, Hammond S, Leisenring WM, Mertens AC, Smith SA, Stovall M, Tucker MA, Weathers RE, Robison LL, Pfeiffer RM (2013) Absolute risk prediction of second primary thyroid cancer among 5-year survivors of childhood cancer. J Clin Oncol 31(1):119–127
Sigurdson AJ, Ronckers CM, Mertens AC, Stovall M, Smith SA, Liu Y, Berkow RL, Hammond S, Neglia JP, Meadows AT, Sklar CA, Robison LL, Inskip PD (2005) Primary thyroid cancer after a first tumour in childhood (the childhood cancer survivor study): a nested case-control study. Lancet 365(9476):2014–2023
Armstrong GT et al (2011) Long-term effects of radiation exposure among adult survivors of childhood cancer: results from the CCSS. Radiat Res 174(6):840–850
Clement SC, Kremer LCM, Verburg FA, Simmons JH, Goldfarb M, Peeters RP, Alexander EK, Bardi E, Brignardello E, Constine LS, Dinauer CA, Drozd VM, Felicetti F, Frey E, Heinzel A, van den Heuvel-Eibrink M, Huang SA, Links TP, Lorenz K, Mulder RL, Neggers SJ, Nieveen van Dijkum E, Oeffinger KC, van Rijn R, Rivkees SA, Ronckers CM, Schneider AB, Skinner R, Wasserman JD, Wynn T, Hudson MM, Nathan PC, van Santen H (2018) Balancing the benefits and harms of thyroid cancer surveillance in survivors of childhood, adolescent and young adult cancer: recommendations from the international Late Effects of Childhood Cancer Guideline Harmonization Group in collaboration with the PanCareSurFup Consortium. Cancer Treat Rev 63:28–39
Veiga LHS, Lubin JH, Anderson H, de Vathaire F, Tucker M, Bhatti P, Schneider A, Johansson R, Inskip P, Kleinerman R, Shore R, Pottern L, Holmberg E, Hawkins MM, Adams MJ, Sadetzki S, Lundell M, Sakata R, Damber L, Neta G, Ron E (2012) A pooled analysis of thyroid cancer incidence following radiotherapy for childhood cancer. Radiat Res 178(4):365–376
Sinnott B, Ron E, Schneider AB (2010) Exposing the thyroid to radiation: a review of its current extent, risks, and implications. Endocr Rev 31(5):756–773
Brodin NP, Rosenschöld PMA, Aznar MC et al (2011) Radiobiological risk estimates of adverse events and secondary cancer for proton and photon radiation therapy of pediatric medulloblastoma. Acta Oncol 50(6):806–816
Tucker MA, Jones PHM, Boice JD et al (1991) Therapeutic radiation at a young age is linked to secondary thyroid cancer. The Late Effects Study Group. Cancer Res:2885–2888
Hudson MM, Mulrooney DA, Bowers DC et al (2009) High-risk populations identified in childhood cancer survivor study investigations: implications for risk-based surveillance. J Clin Oncol 27(14):2405–2414. https://doi.org/10.1200/JCO.2008.21.1516
Bhatti P, Veiga L, Ronckers C (2010) Risk of second primary thyroid cancer after radiotherapy for a childhood cancer in a large cohort study: an update from the childhood cancer survivor study. Radiat Res 174(6):741–752
Children’s Oncology Group (2018) Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancer, version 5.0. www.survivorshipguidelines.org. Accessed October 2018
Gharib H, Papini E, Garber JR et al (2016) American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules – 2016 update. Endocr Pract 22(Supplement 1):1–60
Francis GL, Waguespack SG, Bauer AJ, Angelos P, Benvenga S, Cerutti JM, Dinauer CA, Hamilton J, Hay ID, Luster M, Parisi MT, Rachmiel M, Thompson GB, Yamashita S, American Thyroid Association Guidelines Task Force (2015) Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid 25(7):716–759
Cibas ES, Ali SZ (2017) The 2017 Bethesda system for reporting thyroid cytopathology. Thyroid 27(11):1341–1346
Tessler FN, Middleton WD, Grant EG, Hoang JK (2018) Re: ACR thyroid imaging, reporting and data system (TI-RADS): white paper of the ACR TI-RADS committee. J Am Coll Radiol 15(3):381–382
Coquia SF, Chu LC, Hamper UM (2014) The role of sonography in thyroid cancer. Radio Clin North Am 52(6):1283–1294
Gallo M, Pesenti M, Valcavi R (2003) Ultrasound thyroid nodule measurement: the “gold standard” and its limitations in clinical decision making. Endocr Pract 9(3):194–199
Podda MG, Terenziani M, Gandola L, Collini P, Pizzi N, Marchianò A, Morosi C, Luksch R, Ferrari A, Casanova M, Spreafico F, Polastri D, Meazza C, Catania S, Schiavello E, Biassoni V, Massimino M (2014) Thyroid carcinoma after treatment for malignancies in childhood and adolescence: from diagnosis through follow-up. Med Oncol 31(8):121
Clement SC, Kremer LCM, Links TP, Mulder RL, Ronckers CM, van Eck-Smit B, van Rijn R, van der Pal H, Tissing WJ, Janssens GO, van den Heuvel-Eibrink M, Neggers SJ, van Dijkum E, Peeters RP, van Santen H (2015) Is outcome of differentiated thyroid carcinoma influenced by tumor stage at diagnosis? Cancer Treat Rev 41(1):9–16
Goldfarb M, Freyer DR (2014) Comparison of secondary and primary thyroid cancer in adolescents and young adults. Cancer 120(8):1155–1161
Al Nofal A, Gionfriddo MR, Javed A et al (2016) Accuracy of thyroid nodule sonography for the detection of thyroid cancer in children: systematic review and meta-analysis. Clin Endocrinol 84(3):423–430
Davies L, Welch HG (2014) Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg 140(4):317–322
Polyzos SA, Anastasilakis AD (2009) Clinical complications following thyroid fine-needle biopsy: a systematic review. Clin Endocrinol 71(2):157–165
Brignardello E, Corrias A, Isolato G, Palestini N, Cordero di Montezemolo L, Fagioli F, Boccuzzi G (2008) Ultrasound screening for thyroid carcinoma in childhood cancer survivors: a case series. J Clin Endocrinol Metab 93(12):4840–4843
Brignardello E, Felicetti F, Castiglione A et al (2016) Ultrasound surveillance for radiation-induced thyroid carcinoma in adult survivors of childhood cancer. Eur J Cancer 55(2016):74–80
Li Z, Franklin J, Zelcer S, Sexton T, Husein M (2014) Ultrasound surveillance for thyroid malignancies in survivors of childhood cancer following radiotherapy: a single institutional experience. Thyroid 24(12):1796–1805
Tonorezos ES, Barnea D, Moskowitz CS et al (2017) Screening for thyroid cancer in survivors of childhood and young adult cancer treated with neck radiation. J Cancer Surviv 11(3):302–308
Danylesko I, Shimoni A (2018) Second malignancies after hematopoietic stem cell transplantation. Curr Treat Options in Oncol 19(2)
Kumar RJ, Zhai H, Both S, Tochner Z, Lustig R, Hill-Kayser C (2013) Breast cancer screening for childhood cancer survivors after craniospinal irradiation with protons versus x-rays: a dosimetric analysis and review of the literature. J Pediatr Hematol Oncol 35(6):462–467
Ho ESQ, Barrett SA, Mullaney LM (2017) A review of dosimetric and toxicity modeling of proton versus photon craniospinal irradiation for pediatrics medulloblastoma. Acta Oncol (Madr) 56(8):1031–1042. https://doi.org/10.1080/0284186X.2017.1324207
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hess, J., Schafernak, K., Newbern, D. et al. Ultrasound is superior to palpation for thyroid cancer detection in high-risk childhood cancer and BMT survivors. Support Care Cancer 28, 5117–5124 (2020). https://doi.org/10.1007/s00520-020-05340-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-020-05340-0