Abstract
Background
Diarrhea is one of the most frequent class adverse events associated with targeted oral antineoplastic agents (OAAs). Our objective was to analyze the incidence, characteristics, and severity of diarrhea in cancer patients in clinical practice.
Methods
An observational, longitudinal, and prospective study of cancer outpatients treated with targeted OAAs was carried out in a tertiary hospital. Targed OAAs analyzed were anaplastic lymphoma kinase inhibitors, BCR-ABL inhibitors, cyclin-dependent kinase inhibitors, epidermal growth factor receptor inhibitors, mTOR inhibitors, poly (ADP-ribose) polymerase inhibitors, and vascular endothelial growth factor receptor inhibitors. Patients were given a data collection form to record daily the number, severity (CTCAE version 5.0), and characteristics of stools during the first 30 days of treatment with OAAs. Multivariate analysis was performed to identify risk factors associated with the incidence of diarrhea.
Results
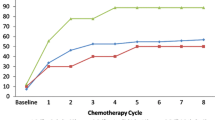
We analyzed 240 patients, of whom 28.7% experienced diarrhea (25.4% grades 1–2 and 3.3% grades 3–4). Patients treated with EGFR and VEGFR inhibitors had a higher incidence of diarrhea. The multivariate analysis revealed that taking the OAA with food was associated with a lower risk of diarrhea (OR = 0.404 [0.205–0.956], p = 0.038).
Conclusions
More than a third of patients in treatment with OAAs presented diarrhea (any grade), and 22.1% of stools were semi-liquid/liquid. In multivariate analysis, taking the OAA on an empty stomach was associated with a statistically significant increase in the incidence of diarrhea.


Similar content being viewed by others
Data availability
N/A
References
IMS Institute for Healthcare Informatics (2016) Global oncology trend report. A review of 2015 and outlook to 2020
Escudero-Vilaplana V, Revuelta-Herrero JL, Collado-Borrell R, Marzal-Alfaro B, Gimenez-Manzorro A, Herranz-Alonso A, Sanjurjo-Saez M (2019) Oral antineoplastic agents: assessment of safety and dose adjustments in clinical practice. Expert Opin Drug Saf 18(9):861–868
Ota K, Takeuchi T, Kojima Y, Harada S, Ozaki H, Sugawara N, Hirata Y, Yamaguchi T, Terazawa T, Kakimoto K, Kii T, Goto M, Higuchi K (2019) Fluorepyrimmide-induced intestinal mucosal injury is associated with the severity of chemotherapy-related diarrhea. Scand J Gastroenterol 54(2):227–232
Pessi MA, Zilembo N, Haspinger ER, Molino L, di Cosimo S, Garassino M, Ripamonti CI (2014) Targeted therapy-induced diarrhea: a review of the literature. Crit Rev Oncol Hematol 90(2):165–179
Rugo HS, Di Palma JA, Tripathy D et al (2019) The characterization, management and future considerations of ErbB-family TKI-associated diarrhea. Breast Cancer Res Treat 175(1):5–15
Andreyev J, Ross P, Donnellan C et al (2014) Guidance on the management of diarrhea during cancer chemotherapy. Lancet Oncol 15(10):e477–e460
Tarricone R, Abu Koush D, Nyanzi-Wakholi B, Medina-Lara A (2016) A systematic literature review of the economic implications of chemotherapy-induced diarrhea and its impact on quality of life. Crit Rev Oncol Hematol 99:37–48
Forde C (2017) Systemic anti-cancer therapy-induced diarrhea. Br J Hosp Med (Lond) 78(9):C135–C139
National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE), Version 5.0. Bethesda, Md. U.S. Department of Health and Human Services, National Institutes of Health, 2017. National Cancer Institute
Richardson G, Dobish R (2007) Chemotherapy induced diarrhea. J Oncol Pharm Pract 13(4):181–189
Aw DC, Tan EH, Chin TM et al (2018) Management of epidermal growth factor receptor tyrosine kinase inhibitor-related cutaneous and gastrointestinal toxicities. Asia Pac J Clin Oncol 14(1):23–31
Davila M, Bresalier RS (2008) Gastrointestinal complications of oncologic therapy. Nat Clin Pract Gastroenterol Hepatol 5(12):682–696
Iacovelli R, Pietrantonio F, Palazzo A, Maggi C, Ricchini F, de Braud F, di Bartolomeo M (2014) Incidence and relative risk of grade 3 and 4 diarrhoea in patients treated with capecitabine or 5-fluorouracil: a meta-analysis of published trials. Br J Clin Pharmacol 78(6):1228–1237
Barcenas CH, Hurvitz SA, Palma JAD et al (2020) Improved tolerability of neratinib in patients with HER2-positive early-stage breast cancer: the CONTROL trial. Ann Oncol 31(9):1223–1230
Xu C, Ravva P, Dang JS, Laurent J, Adessi C, McIntyre C, Meneses-Lorente G, Mercier F (2018) A continuous-time multistate Markov model to describe the occurrence and severity of diarrhea events in metastatic breast cancer patients treated with lumretuzumab in combination with pertuzumab and paclitaxel. Cancer Chemother Pharmacol 82(3):395–406
Cheema PK, Thawer A, Leake J, Cheng SY, Khanna S, Charles Victor J (2019) Multi-disciplinary proactive follow-up algorithm for patients with advanced NSCLC receiving afatinib. Support Care Cancer 27(3):1029–1039
Chan A, Delaloge S, Holmes FA, ExteNET Study Group et al (2016) Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 17(3):367–377
Johnston SRD, Harbeck N, Hegg R et al (2020) Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2-, node-positive, high-risk, early breast cancer (monarchE). J Clin Oncol 20:JCO2002514
Leboulleux S, Bastholt L, Krause T et al (2012) Vandetanib in locally advanced or metastatic differenciated thyroid cancer: a randomized, double-blind, phase 2 trial. Lancet Oncol 123(9):897–905
European Public Assessment Reports. Amsterdam: European Medicines Agency. Available at: https://www.ema.europa.eu/en/medicines/ema_group_types/ema_medicine [Accessed in July 2020]
Passaro A, Di Maio M, Del Signore E et al (2014) Management of nonhematologic toxicities associated with different EGFR-TKIs in advanced NSCLC: a comparison analysis. Clin Lung Cancer 15(4):307–312
Dranitsaris G, Lacouture ME (2014) Development of precition tools for diarrhea and rash in breast cancer patients receiving lapatinib in combination with capcitabine. Breast Cancer Res Treat 147(3):631–638
Carlotto A, Hogsett VL, Maiorini EM, Razulis JG, Sonis ST (2013) The economic burden of toxicities associated with cancer treatment: review of the literature and analysis of nausea, vomiting, diarrhoea, oral mucositis andfatigue. Pharmacoeconomics 31:753–766
Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE), Version 1.0. Bethesda, Md. U.S. Department of Health and Human Services, National Institutes of Health, 2017. National Cancer Institute
Di Maio M, Gallo C, Leighl NB et al (2015) Symptomatic toxicities experienced during anticancer treatment: agreement between patient and physician reporting in three randomized trials. J Clin Oncol 33(8):910–915
Author information
Authors and Affiliations
Contributions
Dr. Martín and Dr. Escudero-Vilaplana conceived the idea. Dr. Escuero-Vilaplana, Dr. Collado-Borrell, Dr. del Monte-Millán, Dr. Gómez, Dr. Revuelta-Herrero, Dr. Hoyo-Muñoz, Dr. Marzal-Alfaro, Dr. Gonzalez-Haba, Dr. Lopez-Tarruella, and Dra. Jerez performed the measurements and collected the data. Dr. Herranz, Dr. Sanjurjo, and Dr. Martín were involved in planning and supervised the work. Dr. Escudero-Vilaplana, Dr. Collado-Borrell, and Dr. del Monte-Millán processed the experimental data, performed the analysis, drafted the manuscript, and designed the figures. Dr. Martín, Dr. Herranz, and Dr. Sanjurjo aided in interpreting the results and worked on the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the local ethics committee (study code MMJ-ITK-2013-01) and conducted in accordance with the ethical principles of the Declaration of Helsinki. Patients signed an informed consent before entering the study and for publication.
Consent for publication
Patients signed an informed consent before entering the study and for publication.
Conflict of interest
Dr. Escudero-Vilaplana received support to continuing education/advisory fees from Amgen, Astellas, AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Ipsen Pharma, Janssen and Merck Sharp & Dohme, Novartis, and Pfizer, outside the submitted work.
Dr. Collado-Borrell received support to continuing education/advisory fees from Boehringer ingelheim, Hoffmann-La Roche, Bristol-Myers Squibb, Janssen Cilag, and Pfizer, outside the submitted work.
Dr. Marzal-Alfaro received support to continuing education/advisory fees from Roche, Abbvie, Pfizer, and Bayer Hispania, outside the submitted work.
Dr. Gonzalez-Haba received support to advisory fees from Bayer, Bristol-Myers Squibb, and Novartis, outside the submitted work.
Dr. Lopez-Tarruella Cobo has received consulting/advisory fees from Celgene, Novartis, Pierre Fabre, Pfizer, Roche, Astra-Zeneca, Eisai, Daiichi-Sankyo, and Lilly and speakers’ honoraria from Lilly, Novartis, and Pfizer, outside the submitted work.
Dr. Jerez Gilarranz has a consultant or advisory role at Novartis, Pfizer, Roche, and AstraZeneca and has received speaker honoraria from Roche, Novartis, and AstraZeneca and travel grants from Roche, Novartis, Pfizer, and Teva.
Dr. Herranz reported honorary/advisory fees from Astellas, Janssen, Kern, and Novartis, outside the submitted work.
Dr. Martin has received research grants from Roche, PUMA, and Novartis; consulting/advisory fees from AstraZeneca, Amgen, Taiho Oncology, Roche/Genentech, Novartis, PharmaMar, Eli Lilly, PUMA, Taiho Oncology, Daiichi Sankyo, and Pfizer; and speakers’ honoraria from AstraZeneca, Lilly, Amgen, Roche/Genentech, Novartis, and Pfizer, outside the submitted work.
Code availability
N/A
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Escudero-Vilaplana, V., Collado-Borrell, R., del Monte-Millán, M. et al. Assessment of diarrhea as side effect of oral targeted antineoplastic agents in clinical practice. Support Care Cancer 29, 4673–4681 (2021). https://doi.org/10.1007/s00520-021-06016-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-021-06016-z