Abstract
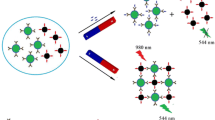
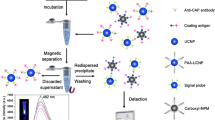
A high-sensitivity fluorescence immunoassay for atrazine was established. It is making use of hydrophilic NaYF4:Yb/Er upconversion nanoparticles (UCNPs) conjugated to the anti-atrazine antibody as signal probe, and of polystyrene magnetic microspheres (PMMs) conjugated to the coating antigen as the capture probe. The coating antigen on the capture probe competes with atrazine for binding to the antibody on the signal probe to form the immuno complex. The complex is separated from the test system by magnetic action, and its green fluorescence is then measured at excitation/emission wavelengths of 980/552 nm using a fluorescence spectrophotometer equipped with an external 980 nm laser. The method was applied to the determination of atrazine in corn, rice, sugar cane juice, and river water. The immunoassay has a linear range that extends from 0.005 to 10 μg·L−1. The assay also recognizes propazine and prometryn, and it therefore can be applied to detect these three herbicides simultaneously. Sugar cane juice and river water samples can be analyzed directly without any pretreatment. The detection limits for atrazine are 2 ng·L−1 in sugar cane juice and river water samples, and 20 ng·kg−1 in corn and rice samples. The recoveries of spiked samples range from 84.7 to 113.6%.

A sensitive fluorescence immunoassay combining magnetic separation for the detection of atrazine in cereal, juice, and water samples using NaYF4:Yb/Er upconversion nanoparticles as labels.




Similar content being viewed by others
References
Iriel A, Novo JM, Cordon GB, Lagorio MG (2014) Atrazine and methyl viologen effects on chlorophyll-a fluorescence revisited-implications in photosystems emission and ecotoxicity assessment. Photochem Photobiol 90:107–112
Czaplicka M, Barchanska H, Jaworek K, Kaczmarczyk B (2018) The interaction between atrazine and the mineral horizon of soil: a spectroscopic study. J Soils Sediments 18:827–834
Nödler K, Licha T, Voutsa D (2013) Twenty years later--atrazine concentrations in selected coastal waters of the Mediterranean and the Baltic Sea. Mar Pollut Bull 70:112–118
Singh S, Kumar V, Chauhan A, Datta S, Wani AB, Singh N, Singh J (2018) Toxicity, degradation and analysis of the herbicide atrazine. Environ Chem Lett 16:211–237
Sun JT, Pan LL, Zhan Y, Tsang DCW, Zhu LZ, Li XD (2017) Atrazine contamination in agricultural soils from the Yangtze River Delta of China and associated health risks. Environ Geochem Health 39:369–378
Ta N, Zhou F, Gao ZQ, Zhong M, Sun C (2006) The status of pesticide residues in the drinking water sources in Meiliangwan Bay, Taihu Lake of China. Environ Monit Assess 123:351–370
Fan WQ, Yanase T, Morinaga H, Condo S, Okabe T, Nomura M, Komatsu T, Morohashi KI, Hayes TB, Takayanagi R, Nawata H (2007) Atrazine-induced aromatase expression is sf-1 dependent: implications for endocrine disruption in wildlife and reproductive cancers in humans. Environ Health Perspect 115:720–727
Zhang XF, Wang MQ, Gao SY, Ren R, Zheng J, Zhang Y (2011) Atrazine-induced apoptosis of splenocytes in BALB/C mice. BMC Med 9:117
Lin J, Zhao HS, Qin L, Li XN, Zhang C, Xia J, Li JL (2018) Atrazine triggers mitochondrial dysfunction and oxidative stress in quail (Coturnix C. coturnix) cerebrum via activating xenobiotic-sensing nuclear receptors and modulating cytochrome p450 systems. J Agric Food Chem 66:6402–6413
Weber GJ, Sepúlveda MS, Peterson SM, Lewis SS, Freeman JL (2013) Transcriptome alterations following developmental atrazine exposure in zebrafish are associated with disruption of neuroendocrine and reproductive system function, cell cycle, and carcinogenesis. Toxicol Sci 132:458–466
Jia LC, Su M, Wu XQ, Sun HW (2016) Rapid selective accelerated solvent extraction and simultaneous determination of herbicide atrazine and its metabolites in fruit by ultra high performance liquid chromatography. J Sep Sci 39:4512–4519
Hu ED, Cheng HF (2013) Rapid extraction and determination of atrazine and its degradation products from microporous mineral sorbents using microwave-assisted solvent extraction followed by ultra-HPLC-MS/MS. Microchim Acta 180:703–710
Djozan D, Ebrahimi B (2008) Preparation of new solid phase micro extraction fiber on the basis of atrazine-molecular imprinted polymer: application for GC and GC/MS screening of triazine herbicides in water, rice and onion. Anal Chim Acta 616:152–159
Rubira RJG, Camacho SA, Aoki PHB, Maximino MD, Alessio P, Martin CS, Oliveira ON Jr, Fatore FM, Paulovich FV, Constantino CJL (2014) Detection of trace levels of atrazine using surface-enhanced Raman scattering and information visualization. Colloid Polym Sci 292:2811–2820
Islam K, Chand R, Han D, Kim YS (2015) Microchip capillary electrophoresis based electroanalysis of triazine herbicides. Bull Environ Contam Toxicol 94:41–45
Liu GY, Yang X, Li TF, Yu HL, Du XW, She YX, Wang J, Wang SS, Jin F, Jin MJ, Shao H, Zheng LF, Zhang YX, Zhou P (2015) Spectrophotometric and visual detection of the herbicide atrazine by exploiting hydrogen bond-induced aggregation of melamine-modified gold nanoparticles. Microchim Acta 182:1983–1989
Infante CMC, de Pra’ Urio R, Masini JC (2011) Improving the detectability of sequential injection chromatography (sic): determination of triazines by exploiting liquid core waveguide (lcw) detection. Anal Lett 44:503–513
Kaur J, Boro RC, Wangoo N, Singh KR, Suri CR (2008) Direct hapten coated immunoassay format for the detection of atrazine and 2,4-dichlorophenoxyacetic acid herbicides. Anal Chim Acta 607:92–99
Na Y, Sheng W, Yuan M, Li LL, Liu B, Zhang Y, Wang S (2012) Enzyme-linked immunosorbent assay and immunochromatographic strip for rapid detection of atrazine in water samples. Microchim Acta 177:177–184
Sai N, Sun WJ, Wu YT, Sun Z, Yu GG, Huang GW (2016) A highly sensitive immunoassay for atrazine based on covalently linking the small molecule hapten to a urea–glutaraldehyde network on a polystyrene surface. Int Immunopharmacol 40:480–486
Qie ZW, Bai JL, Xie B, Yuan L, Song N, Peng Y, Fan XJ, Zhou HY, Chen FC, Li S, Ning BA, Gao ZX (2015) Sensitive detection of atrazine in tap water using TELISA. Analyst 140:5220–5226
Shim WB, Yang ZY, Kim JY, Choi JG, Je JH, Kang SJ, Kolosova AY, Eremin SA, Chung DH (2006) Immunochromatography using colloidal gold−antibody probe for the detection of atrazine in water samples. J Agric Food Chem 54:9728–9734
Hendrickson OD, Skopinskaya SN, Yarkov SP, Zherdev AV, Dzantiev BB (2004) Development of liposome immune lysis assay for the herbicide atrazine. J Immunoass Immunochem 25:279–294
Chouhan RS, Rana KVS, Suri CR, Thampi RK, Thakur MS (2010) Trace-level detection of atrazine using immuno-chemiluminescence: dipstick and automated flow injection analyses formats. J AOAC Int 93:28–35
Liu X, Li WJ, Li L, Yang Y, Mao LG, Peng Z (2014) A label-free electrochemical immunosensor based on gold nanoparticles for direct detection of atrazine. Sensors Actuators B Chem 191:408–414
Yang M, Zhao XB, Zheng S, Liu X, Jin BW, Li H, Gan Y (2017) A new electrochemical platform for ultrasensitive detection of atrazine based on modified self-ordered Nb2O5 nanotube arrays. J Electroanal Chem 791:17–22
Farré M, Martínez E, Ramón J, Navarro A, Radjenovic J, Mauriz E, Lechuga L, Marco MP, Barceló D (2007) Part per trillion determination of atrazine in natural water samples by a surface plasmon resonance immunosensor. Anal Bioanal Chem 388:207–214
Rodriguez-Mozaz S, Reder S, de Alda ML, Gauglitz G, Barceló D (2004) Simultaneous multi-analyte determination of estrone, isoproturon and atrazine in natural waters by the river analyser (RIANA), an optical immunosensor. Biosens Bioelectron 19:633–640
Feng J, Shan GM, Maquieira A, Koivunen ME, Guo B, Hammock BD, Kennedy IM (2003) Functionalized europium oxide nanoparticles used as a fluorescent label in an immunoassay for atrazine. Anal Chem 75:5282–5286
Seidel M, Dankbar DM, Gauglitz G (2004) A miniaturized heterogeneous fluorescence immunoassay on gold-coated nano-titer plates. Anal Bioanal Chem 379:904–912
Wegner KD, Lindén S, Jin ZW, Jennings TL, el Khoulati R, van Bergen en Henegouwen PMP, Hildebrandt N (2014) Nanobodies and nanocrystals: highly sensitive quantum dot-based homogeneous FRET immunoassay for serum-based egfr detection. Small 10:734–740
Wang M, Abbineni G, Clevenger A, Mao CB, Xu SK (2011) Upconversion nanoparticles: synthesis, surface modification and biological applications. Nanomedicine 7:710–729
Hua XD, You HJ, Luo PW, Tao ZX, Chen H, Liu FQ, Wang MH (2017) Upconversion fluorescence immunoassay for imidaclothiz by magnetic nanoparticle separation. Anal Bioanal Chem 409:6885–6892
Hu GS, Sheng W, Zhang Y, Wu XN, Wang S (2015) A novel and sensitive fluorescence immunoassay for the detection of fluoroquinolones in animal-derived foods using upconversion nanoparticles as labels. Anal Bioanal Chem 407:8487–8496
Liu CH, Wang Z, Wang XK, Li ZP (2011) Surface modification of hydrophobic NaYF4:Yb,Er upconversion nanophosphors and their applications for immunoassay. Sci China Chem 54:1292–1297
Acknowledgments
This work was supported by the Open Project Program of State Key Laboratory of Food Nutrition and Safety, Tianjin University of Science and Technology (Project No. SKLFNS-KF-201819), the Tianjin Municipal Science and Technology Commission (Project No. 16PTSYJC00130), the National Key R and D Program of China (Project No. 2016YFD0401204), and the International Science and Technology Cooperation Program of China (Project No. 2014DFR30350).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 537 kb)
Rights and permissions
About this article
Cite this article
Sheng, W., Shi, Y., Ma, J. et al. Highly sensitive atrazine fluorescence immunoassay by using magnetic separation and upconversion nanoparticles as labels. Microchim Acta 186, 564 (2019). https://doi.org/10.1007/s00604-019-3667-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3667-3