Abstract
A solid-phase extraction method is presented for micro-extraction of three progestins (levonorgestrel, 19-norethisterone acetate and medroxyprogesterone acetate) from water samples. A mini-column was packed with 60 mg of oxidized multiwalled carbon nanotubes and coupled to a flow injection assembly. The extraction parameters, such as washing solution, eluent type, eluent volume, flow rate and sample volume, were optimized. Separation and determination were performed by HPLC with UV detection. The method has a good linear range (0.90–9.0 μg L−1), acceptable limits of detection (0.05–0.14 μg L−1) and low RSDs (0.8–4.6%). Attractive features of the method include low consumption of organic solvents and preconcentration factors of up to 100. The method was applied to analyze stream, underground and effluent water samples, and recoveries between 74 and 121% were obtained.

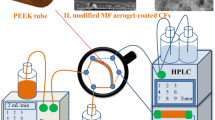
Schematic representation of the flow injection assembly couples to an ox-MWCNTs extraction column used to perform the solid phase extraction procedure of progestins in environmental water samples.



Similar content being viewed by others
References
Anastas N. Overview of chemicals of emerging concern. To be presented at PCWWA November meeting, Halifax, MA, November 18, 2015
Naidu R, Arias Espana V, Liu Y, Jit J (2016) Emerging contaminants in the environment: risk-based analysis for better management. Chemosphere 154:350–357. https://doi.org/10.1016/j.chemosphere.2016.03.068
Yang L, Luan T, Lan C (2006) Solid-phase microextraction with on-fiber silylation for simultaneous determinations of endocrine disrupting chemicals and steroid hormones by gas chromatography–mass spectrometry. J Chromatogr A 1104:23–32. https://doi.org/10.1016/j.chroma.2005.11.108
Scaglia H, Chichizola C, Franconi MC, Ludueña B, Mastandrea C, Scaglia J (2009) Disruptores endocrinos. Composición química, mecanismo de acción y efecto sobre el eje reproductivo. Reproducción 24:74–86
Schindler AE, Campagnoli C, Druckmann R, Huber J, Pasqualini JR, Schweppe KW, Thijssen JHH (2003) Classification and pharmacology of progestins. Maturitas 46:7–16. https://doi.org/10.1016/j.maturitas.2003.09.014
Fent K (2015) Progestins as endocrine disrupters in aquatic ecosystems: concentrations, effects and risk assessment. Environ Int 84:115–130. https://doi.org/10.1016/j.envint.2015.06.012
Tomsíková H, Aufartová J, Solich P, Sosa-Ferrera Z, Santana-Rodríguez JJ, Novákova L (2012) High-sensitivity analysis of female-steroid hormones in environmental samples. Trends Anal Chem 34:35–58. https://doi.org/10.1016/j.trac.2011.11.008
Majors RE (2010) Solid-phase extraction. In: Pawliszyn J, Lord HL (eds) Handbook of Sample Preparation. Wiley & Sons, Inc, pp 53–79
Aufartová J, Torres-Padrón ME, Sosa-Ferrera Z, Solich P, Santana-Rodrguez JJ (2012) Optimisation of an in-tube solid phase microextraction method coupled with HPLC for determination of some oestrogens in environmental liquid samples using different capillary columns. Int J Environ Anal Chem 92:382–396. https://doi.org/10.1080/03067319.2011.585714
Vakondios N, Mazioti AA, Koukouraki EE, Diamadopoulos E (2016) An analytical method for measuring specific endocrine disruptors in activated sludge (biosolids) using solid phase microextraction-gas chromatography. J Environ Chem Eng 4:1910–1917. https://doi.org/10.1016/j.jece.2016.03.018
Liu SS, Ying GG, Liu S, Lai HJ, Chen ZF, Pan CG, Zhao JL, Chen J (2014) Analysis of 21 progestagens in various matrices by ultra-high-performance liquid chromatography tandem mass spectrometry (UHPLC-MS/MS) with diverse sample pretreatment. Anal Bioanal Chem 406:7299–7311. https://doi.org/10.1007/s00216-014-8146-4
Shen X, Chang H, Sun D, Wang L, Wu F (2018) Trace analysis of 61 natural and synthetic progestins in river water and sewage effluents by ultra-high performance liquid chromatography tandem mass spectrometry. Water Res 133:142–152. https://doi.org/10.1016/j.watres.2018.01.030
Houtman CJ, Broek R, Brouwer A (2018) Steroid hormonal bioactivities, culprit natural and synthetic hormones and other emerging contaminants in waste water measured using bioassays and UPLC-tQ-MS. Sci Total Environ 630:1492–1501. https://doi.org/10.1016/j.scitotenv.2018.02.273
Huysman S, Van Meulebroek L, Vanryckeghem F, Van Langenhove H, Demeestere K, Vanhaecke L (2017) Development and validation of an ultra-high performance liquid chromatographic high resolution Q-Orbitrap mass spectrometric method for the simultaneous determination of steroidal endocrine disrupting compounds in aquatic matrices. Anal Chim Acta 984:140–150. https://doi.org/10.1016/j.aca.2017.07.001
Golovko O, Šauer P, Fedorova G, Kocour Kroupová H, Grabic R (2018) Determination of progestogens in surface and waste water using SPE extraction and LC- APCI/APPI-HRPS. Sci Total Environ 621:1066–1073. https://doi.org/10.1016/j.scitotenv.2017.10.120
Sun L, Yong W, Chu X, Lin JM (2009) Simultaneous determination of 15 steroidal oral contraceptives in water using solid-phase disk extraction followed by high performance liquid chromatography–tandem mass spectrometry. J Chromatogr A 1216:5416–5423. https://doi.org/10.1016/j.chroma.2009.05.041
Ulusoy H, Yılmaz E, Soylak M (2019) Magnetic solid phase extraction of trace paracetamol and caffeine in synthetic urine and wastewater samples by a using core shell hybrid material consisting of graphene oxide/multiwalled carbon nanotube/Fe3O4/SiO2. Microchem J 145:843–885. https://doi.org/10.1016/j.microc.2018.11.056
Płotka-Wasylka J, Szczepańska N, de la Guardia M, Namieśni J (2016) Modern trends in solid phase extraction: new sorbent media. Trends Anal Chem 77:23–43. https://doi.org/10.1016/j.trac.2015.10.010
Soylak M, Emre Unsal Y (2010) Chromium and iron determinations in food and herbal plant samples by atomic absorption spectrometry after solid phase extraction on single-walled carbon nanotubes (SWCNTs) disk. Food Chem Toxicol 48:1511–1515. https://doi.org/10.1016/j.fct.2010.03.017
Sahmetlioglu E, Yilmaz E, Aktas E, Soylak M (2014) Polypyrrole/multi-walled carbon nanotube composite for the solid phase extraction of lead(II) in water samples. Talanta 119:447–451. https://doi.org/10.1016/j.talanta.2013.11.044
Dil EA, Asfaram A, Sadeghfar F (2019) Magnetic dispersive micro-solid phase extraction with the CuO/ZnO@Fe3O4-CNTs nanocomposite sorbent for the rapid pre-concentration of chlorogenic acid in the medical extract of plants, food, and water samples. Analyst 144:2684–2695. https://doi.org/10.1039/c8an02484g
Ebrahimpour B, Yamini Y, Seidi S, Tajik M (2015) Nano polypyrrole-coated magnetic solid phase extraction followed by dispersive liquid phase microextraction for trace determination of megestrol acetate and levonorgestrel. Anal Chim Acta 885:98–105. https://doi.org/10.1016/j.aca.2015.05.025
Es’haghi Z, Nezhadali A, Khatibi AD (2016) Magnetically responsive polycaprolactone nanoparticles for progesterone screening in biological and environmental samples using gas chromatography. Anal Bioanal Chem 408:37–49. https://doi.org/10.1007/s00216-016-9650-5
Vasconcelos I, Fernandes C (2017) Magnetic solid phase extraction for determination of drugs in biological matrices. Trends Anal Chem 89:41–52. https://doi.org/10.1016/j.trac.2016.11.011
Su R, Wang X, Xu X, Wang Z, Li D, Zhao X, Li X, Zhang H, Yu A (2011) Application of multiwall carbon nanotubes-based matrix solid phase dispersion extraction for determination of hormones in butter by gas chromatography mass spectrometry. J Chromatogr A 1218:5047–5054. https://doi.org/10.1016/j.chroma.2011.05.088
Cerdà V, Ferrer L, Avivar J, Cerdà A (2014) A. Flow analysis. A practical guide, 1st edn. Elsevier Science
Valcárcel M, Cárdenas S (2005) Vanguard-rearguard analytical strategies. Trends Anal Chem 24:67–74. https://doi.org/10.1016/j.trac.2004.07.016
Xiong X, Ouyang J, Baeyens WRG, Delanghe JR, Shen X, Yang Y (2006) Enhanced separation of purine and pyrimidine bases using carboxylic multiwalled carbon nanotubes as additive in capillary zone electrophoresis. Electrophoresis 27:3243–3253. https://doi.org/10.1002/elps.200500870
AOAC Peer-Verified Methods Program, Manual on policies and procedures, Arlington, VA, USA, 1998
Augusto F, Hantao L, Mogollón N, Braga S (2013) New materials and trends in sorbents for solid-phase extraction. Trends Anal Chem 43:14–23. https://doi.org/10.1016/j.trac.2012.08.012
Li Q, Wang X, Yuan D (2009) Solid-phase extraction of polar organophosphorous pesticides from aqueous samples with oxidized carbon nanotubes. J Environ Monit 11:439–444. https://doi.org/10.1039/b816271a
El-Sheikh AH, Insisi AA, Sweileh JA (2007) Effect of oxidation and dimensions of multi- walled carbon nanotubes on solid phase extraction and enrichment of some pesticides from environmental waters prior to their simultaneous determination by high performance liquid chromatography. J Chromatogr A 1164:25–32. https://doi.org/10.1016/j.chroma.2007.07.009
Herrera-Herrera AV, Ravelo-Pérez LM, Hernández-Borges J, Afonso MM, Palenzuela JA, Roríguez-Delgado MA (2011) Oxidized multi-walled carbon nanotubes for the dispersive solid- phase extraction of quinolone antibiotics from water samples using capillary electrophoresis and large volume sample stacking with polarity switching. J Chromatogr A 1218:5352–5361. https://doi.org/10.1016/j.chroma.2011.06.031
Chen S, Liu C, Yang M, Lu D, Zhu L, Wang Z (2009) Solid-phase extraction of Cu, Co and Pb on oxidized single-walled carbon nanotubes and their determination by inductively coupled plasma mass spectrometry. J Hazard Mater 170:247–251. https://doi.org/10.1016/j.jhazmat.2009.04.104
Herrero-Latorre C, Barciela-García J, García-Martín S, Pena-Crecente RM (2018) Graphene and carbon nanotubes as solid phase extraction sorbents for the speciation of chromium: a review. Anal Chim Acta 1002:1–17. https://doi.org/10.1016/j.aca.2017.11.042
Massart DL (1997) Handbook of Chemometrics and Qualimetrics, 1st edn. Elsevier Science
Acknowledgments
Authors gratefully acknowledge to Universidad Nacional del Sur (PGI 24/ZQ17) and Comisión Nacional de Investigaciones Científicas y Técnicas (CONICET).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 177 kb)
Rights and permissions
About this article
Cite this article
Aguinaga Martínez, M.V., Llamas, N.E., Ávila Orozco, F.D. et al. Oxidized carbon nanotubes as sorbent for miniaturized solid-phase extraction of progestins from environmental water samples prior to their determination by HPLC-UV. Microchim Acta 187, 153 (2020). https://doi.org/10.1007/s00604-020-4116-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-4116-z