Abstract
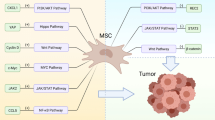
The development of new therapies based on tumor biology is one of the main topics in cancer treatment. In this regard, investigating the microenvironment and cellular composition of the tumor is of particular interest. Mesenchymal stem cells (MSCs) are a major group of cells in the tumor tissue and play a critical role in tumor growth and development. Investigating the mechanisms by which MSCs influence tumor growth and progression is very useful in establishing new therapeutic approaches. MSCs have some immunological capacities, including anti-inflammatory, immune-regulatory, and immune-suppressive abilities, which help the tumor growth in the inflammatory condition. They can suppress the proliferation and activation of CD4 + T cells and direct them toward the regulatory phenotype through the release of some factors such as indoleamine 2,3-dioxygenase, prostaglandin E2, and HO-1, PD-1 ligands (PD-L1 and PD-L2) and promote tolerance and apoptosis. Besides, these cells are able to produce adenosine. Adenosine has a key role in controlling the immune system by signaling through receptors located on the surface of immune cells. It plays a very essential role in tumor growth and progression. In the present review, we investigate and introduce adenosine-producing mesenchymal stem cells as a potential target for cancer treatment.

Similar content being viewed by others
References
Albini A, Sporn MB. The tumour microenvironment as a target for chemoprevention. Nat Rev Cancer. 2007;7(2):139–47.
Rohban R, Pieber TR. Mesenchymal stem and progenitor cells in regeneration: tissue specificity and regenerative potential. Stem Cells Int. 2017. https://doi.org/10.1155/2017/5173732.
Han Y, et al. Mesenchymal stem cells for regenerative medicine. Cells. 2019;8(8):886.
Guerrouahen BS, et al. Enhancing mesenchymal stromal cell immunomodulation for treating conditions influenced by the immune system. Stem cells Int. 2019. https://doi.org/10.1155/2019/7219297.
Poggi A, Giuliani M. Mesenchymal stromal cells can regulate the immune response in the tumor microenvironment. Vaccines. 2016;4(4):41.
Melief SM, et al. Multipotent stromal cells induce human regulatory T cells through a novel pathway involving skewing of monocytes toward anti-inflammatory macrophages. Stem Cells. 2013;31(9):1980–91.
Mougiakakos D, et al. The impact of inflammatory licensing on heme oxygenase-1–mediated induction of regulatory T cells by human mesenchymal stem cells. Blood. 2011;117(18):4826–35.
Davies LC, et al. Mesenchymal stromal cell secretion of programmed death-1 ligands regulates T cell mediated immunosuppression. Stem cells. 2017;35(3):766–76.
Zhang B. CD73: a novel target for cancer immunotherapy. Can Res. 2010;70(16):6407–11.
Young A, et al. Targeting cancer-derived adenosine: new therapeutic approaches. Cancer Discov. 2014;4(8):879–88.
Arab S, et al. Increased efficacy of a dendritic cell–based therapeutic cancer vaccine with adenosine receptor antagonist and CD73 inhibitor. Tumor Biol. 2017;39(3):1010428317695021.
Karnoub AE, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449(7162):557–63.
Shi Y, et al. Tumour-associated mesenchymal stem/stromal cells: emerging therapeutic targets. Nat Rev Drug Discov. 2017;16(1):35–52.
Ren G, et al. Tumor resident mesenchymal stromal cells endow naive stromal cells with tumor-promoting properties. Oncogene. 2014;33(30):4016–20.
Ridge SM, Sullivan FJ, Glynn SA. Mesenchymal stem cells: key players in cancer progression. Mol cancer. 2017;16(1):31.
Krueger TE, et al. Tumor-infiltrating mesenchymal stem cells: Drivers of the immunosuppressive tumor microenvironment in prostate cancer? Prostate. 2019;79(3):320–30.
Li W, et al. Gastric cancer-derived mesenchymal stem cells prompt gastric cancer progression through secretion of interleukin-8. J Exp Clin Cancer Res. 2015;34(1):1–15.
Kansy BA, et al. The bidirectional tumor-mesenchymal stromal cell interaction promotes the progression of head and neck cancer. Stem Cell Res Ther. 2014;5(4):95.
Shahar T, et al. Percentage of mesenchymal stem cells in high-grade glioma tumor samples correlates with patient survival. Neuro-oncology. 2017;19(5):660–8.
Qi J, et al. Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth through hedgehog signaling pathway. Cell Physiol Biochem. 2017;42(6):2242–54.
Wang S, et al. Exosomes secreted by mesenchymal stromal/stem cell-derived adipocytes promote breast cancer cell growth via activation of Hippo signaling pathway. Stem Cell Res Ther. 2019;10(1):117.
Thiery JP. Epithelial–mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2(6):442–54.
Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Investig. 2009;119(6):1420–8.
Tang H, et al. The metastatic phenotype shift toward myofibroblast of adipose-derived mesenchymal stem cells promotes ovarian cancer progression. Carcinogenesis. 2020;41(2):182–93.
Valeta-Magara A, et al. Inflammatory breast cancer promotes development of M2 tumor-associated macrophages and cancer mesenchymal cells through a complex chemokine network. Can Res. 2019;79(13):3360–71.
Lytle NK, Barber AG, Reya T. Stem cell fate in cancer growth, progression and therapy resistance. Nat Rev Cancer. 2018;18(11):669–80.
Takebe N, et al. Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways. Nat Rev Clin oncol. 2011;8(2):97.
Rahmatizadeh F, et al. Bidirectional and opposite effects of naïve mesenchymal stem cells on tumor growth and progression. Adv Pharm Bull. 2019;9(4):539.
Lee HY, Hong IS. Double-edged sword of mesenchymal stem cells: cancer-promoting versus therapeutic potential. Cancer Sci. 2017;108(10):1939–46.
Valkenburg KC, de Groot AE, Pienta KJ. Targeting the tumour stroma to improve cancer therapy. Nat Rev Clin Oncol. 2018;15(6):366–81.
Yin L, et al. Gastric-cancer-derived mesenchymal stem cells: a promising target for resveratrol in the suppression of gastric cancer metastasis. Hum Cell. 2020;33:652–62.
Stagg J, et al. Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc Natl Acad Sci. 2010;107(4):1547–52.
Perrot I, et al. Blocking antibodies targeting the CD39/CD73 immunosuppressive pathway unleash immune responses in combination cancer therapies. Cell Rep. 2019;27(8):2411–25.
Liu H, Xia Y. Beneficial and detrimental role of adenosine signaling in diseases and therapy. J Appl Physiol. 2015;119(10):1173–82.
Borea PA, et al. Pharmacology of adenosine receptors: the state of the art. Physiol Rev. 2018;98(3):1591–625.
Katsuta E, et al. High CD73 expression, regulated by estrogen signaling, associates with cancer aggressiveness in estrogen receptor (+) breast cancer. Cancer Res. 2019;79(13 Supplement):5200. https://doi.org/10.1158/1538-7445.AM2019-5200.
Ono K, et al. Immunohistochemical CD73 expression status in gastrointestinal neuroendocrine neoplasms: a retrospective study of 136 patients. Oncol Lett. 2018;15(2):2123–30.
Mandapathil M, et al. CD73 expression in lymph node metastases in patients with head and neck cancer. Acta Otolaryngol. 2018;138(2):180–4.
Lupia M, et al. CD73 regulates stemness and epithelial-mesenchymal transition in ovarian cancer-initiating cells. Stem Cell Rep. 2018;10(4):1412–25.
Jiang T, et al. Comprehensive evaluation of NT5E/CD73 expression and its prognostic significance in distinct types of cancers. BMC cancer. 2018;18(1):1–10.
Stone JK, et al. Hypoxia induces cancer cell-specific chromatin interactions and increases MALAT1 expression in breast cancer cells. J Biol Chem. 2019;294(29):11213–24.
Li J, et al. CD39/CD73 upregulation on myeloid-derived suppressor cells via TGF-β-mTOR-HIF-1 signaling in patients with non-small cell lung cancer. Oncoimmunology. 2017;6(6):e1320011.
Kheshtchin N, et al. Inhibition of HIF-1α enhances anti-tumor effects of dendritic cell-based vaccination in a mouse model of breast cancer. Cancer Immunol Immunother. 2016;65(10):1159–67.
Antonioli L, et al. CD39 and CD73 in immunity and inflammation. Trends in Mol Med. 2013;19(6):355–67.
Schneider E, et al. Generation and function of non-cell-bound CD73 in inflammation. Front Immunol. 2019;10:1729.
Ujházy P, et al. Evidence for the involvement of ecto-5′-nucleotidase (CD73) in drug resistance. Int J Cancer. 1996;68(4):493–500.
Sajadpoor Z, et al. Valproic acid promotes apoptosis and cisplatin sensitivity through downregulation of H19 noncoding RNA in ovarian A2780 cells. Appl Biochem Biotechnol. 2018;185(4):1132–44.
Khayami R, et al. Role of adenosine signaling in the pathogenesis of head and neck cancer. J Cell Biochem. 2018;119(10):7905–12.
Asgharzade S, et al. The effect of oleuropein on apoptotic pathway regulators in breast cancer cells. Eur J Pharm. 2020;886:173509.
Amini-Farsani Z, et al. MiR-221/222 promote chemoresistance to cisplatin in ovarian cancer cells by targeting PTEN/PI3K/AKT signaling pathway. Cytotechnology. 2018;70(1):203–13.
Arab S, Hadjati J. Adenosine blockage in tumor microenvironment and improvement of cancer immunotherapy. Immune Netw. 2019;19(4):e23.
Jadidi-Niaragh F, et al. Downregulation of CD73 in 4T1 breast cancer cells through siRNA-loaded chitosan-lactate nanoparticles. Tumor Biol. 2016;37(6):8403–12.
Gharibi B, et al. Adenosine receptor subtype expression and activation influence the differentiation of mesenchymal stem cells to osteoblasts and adipocytes. J Bone Miner Res. 2011;26(9):2112–24.
Sattler C, et al. Inhibition of T-cell proliferation by murine multipotent mesenchymal stromal cells is mediated by CD39 expression and adenosine generation. Cell Transpl. 2011;20(8):1221–30.
Saldanha-Araujo F, et al. Mesenchymal stromal cells up-regulate CD39 and increase adenosine production to suppress activated T-lymphocytes. Stem Cell Res. 2011;7(1):66–74.
Chen X, et al. CD73 pathway contributes to the immunosuppressive ability of mesenchymal stem cells in intraocular autoimmune responses. Stem Cells Dev. 2016;25(4):337–46.
Ávila-Ibarra LR, et al. Mesenchymal stromal cells derived from normal cervix and cervical cancer tumors increase CD73 expression in cervical cancer cells through TGF-β1 production. Stem Cells Dev. 2019;28(7):477–88.
Samanta D, et al. Chemotherapy induces enrichment of CD47+/CD73+/PDL1+ immune evasive triple-negative breast cancer cells. Proc Natl Acad Sci. 2018;115(6):E1239–48.
Ayob AZ, Ramasamy TS. Cancer stem cells as key drivers of tumour progression. J Biomed Sci. 2018;25(1):1–18.
Yoe J, et al. Capicua restricts cancer stem cell-like properties in breast cancer cells. Oncogene. 2020;39(17):3489–506.
Simón-Carrasco L, et al. The Capicua tumor suppressor: a gatekeeper of Ras signaling in development and cancer. Cell Cycle. 2018;17(6):702–11.
Xu M, et al. (2018) Role of p38γ MAPK in regulation of EMT and cancer stem cells. Biochimica et Biophysica Acta (BBA)-Mol Basis Dis. 1864;11:3605–17.
Xie C, et al. Tobacco smoke induced hepatic cancer stem cell-like properties through IL-33/p38 pathway. J Exp Clin Cancer Res. 2019;38(1):1–13.
Yagi H, Kitagawa Y. The role of mesenchymal stem cells in cancer development. Fron Genet. 2013;4:261.
Tian K, et al. p38 MAPK contributes to the growth inhibition of leukemic tumor cells mediated by human umbilical cord mesenchymal stem cells. Cell Physiol Biochem. 2010;26(6):799–808.
Acknowledgments
This study was supported by the deputy of Shahrekord University of Medical Sciences and Semnan University of medical sciences.
Funding
This study was supported by a grant from Semnan University of Medical Sciences (Grant number: 1614).
Author information
Authors and Affiliations
Contributions
All authors collaborated in the writing of the manuscript. Samira Asgharzade and Akram Alizadeh wrote the draft of manuscript, and Samaneh Arab was the corresponding author who edited the final version of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethical approval
This paper is a review article and has not received ethical approval.
Informed consent
This paper is a review article, and there was no need for informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arab, S., Alizadeh, A. & Asgharzade, S. Tumor-resident adenosine-producing mesenchymal stem cells as a potential target for cancer treatment. Clin Exp Med 21, 205–213 (2021). https://doi.org/10.1007/s10238-020-00674-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-020-00674-9