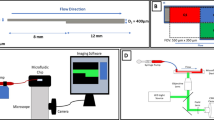
Abstract
When using artificial surfaces that come into contact with the bloodstream, it is important to consider the undesirable consequences of thrombus formation and embolization. Although great progress has been made by creating new surfaces and antithrombotic coatings or evaluating flow conditions, unexpected platelet adhesion and aggregation can lead to the sudden formation of an adverse thrombus. Our experiments in a stagnation point flow chamber with citrate-anticoagulated whole blood and ADP-stimulated platelets mimicked the situations of implanted artificial organs, e.g., mechanical circulatory support devices, or extravascular circulation. With video microscopy, real-time platelet characteristics were observed at shear rate levels between 50 and 500 s−1 on glass, von Willebrand factor, and polyurethane surfaces for at least 5 min after the first contact. Platelet adhesion and aggregation were observed with distinctness in aggregate size, surface coverage, aggregate size, probability of an embolic event, and platelet contraction. The probability of an embolic event increased at lower flow rates. Additionally, platelet contraction was affected by the flow rate. Raising the flow rate intensified the platelet contraction. With this setup, the microembolization caused by surface contact and flow and platelet contraction can be detected in a real-time direct observation. This capability addresses both technical and clinical issues, such as thrombus and embolus formation, and may improve the research on the hemocompatibility of biomaterials.







Similar content being viewed by others
Abbreviations
- ADP:
-
Adenosine diphosphate
- BSA:
-
Bovine serum albumin
- CFD:
-
Computational fluid dynamics
- KCl:
-
Potassium chloride
- MCSD:
-
Mechanical circulatory support devices
- NaCl:
-
Sodium chloride
- NaH2PO4 :
-
Sodium dihydrogen phosphate
- PBS:
-
Phosphate-buffered saline
- PPACK:
-
H-D-Phe-Pro-Arg-chloromethylketone trifluoroacetate
- PRP:
-
Platelet-rich plasma
- VWF:
-
von Willebrand factor
References
Arjun GN, Ramesh P (2012) Structural characterization, mechanical properties, and in vitro cytocompatibility evaluation of fibrous polycarbonate urethane membranes for biomedical applications. J Biomed Mater Res A 100A(11):3042–3050
Backes D, van den Bergh WM, van Duijn AL, Lahpor JR, van Dijk D, Slooter AJC (2012) Cerebrovascular complications of left ventricular assist devices. Eur J Cardiothorac Surg 42(4):612–620
Bang NU, Trygstad CW, Heiden R (1972) Plasma-protein requirements for human platelet aggregation. Ann N Y Acad Sci 201:280
Beiras-Fernandez A, Kanzler I, Michel S, Sadoni S, Kilger E, Beiras A, Kur F (2013) Platelet factor 4-positive thrombi adhering to the ventricles of a ventricular assist device in patients with heparin-induced thrombocytopenia type II. Transplant Proc 45(5):2013–2016
Born GVR (1962) Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature 194(4832):927
Brass LF, Wannemacher KM, Ma P, Stalker TJ (2011) Regulating thrombus growth and stability to achieve an optimal response to injury. J Thromb Haemost 9:66–75
Broos K, Feys HB, De Meyer SF, Vanhoorelbeke K, Deckmyn H (2011) Platelets at work in primary hemostasis. Blood Rev 25(4):155–167
Claesson PM, Blomberg E, Froberg JC, Nylander T, Arnebrant T (1995) Protein interactions at solid–surfaces. Adv Colloid Interface Sci 57:161–227
Clark RE, Brillman J, Davis DA, Lovell MR, Price TRP, Magovern GJ (1995) Microemboli during coronary-artery bypass-grafting—genesis and effect an outcome. J Thorac Cardiovasc Surg 109(2):249–258
Colace TV, Tormoen GW, McCarty OJT, Diamond SL (2013) Microfluidics and coagulation biology. Annu Rev Biomed Eng 15:283–303
Fare S, Valtulina V, Petrini P, Alessandrini E, Pietrocola G, Tanzi MC, Speziale P, Visai L (2005) In vitro interaction of human fibroblasts and platelets with a shape-memory polyurethane. J Biomed Mater Res A 73A(1):1–11
Flamm MH, Diamond SL (2012) Multiscale systems biology and physics of thrombosis under flow. Ann Biomed Eng 40(11):2355–2364
Garcia AJ (2005) Get a grip: integrins in cell-biomaterial interactions. Biomaterials 26(36):7525–7529
Handa H, Major TC, Brisbois EJ, Amoako KA, Meyerhoff ME, Bartlett RH (2014) Hemocompatibility comparison of biomedical grade polymers using rabbit thrombogenicity model for preparing nonthrombogenic nitric oxide releasing surfaces. J Mater Chem B 2(8):1059–1067
Hathcock JJ (2006) Flow effects on coagulation and thrombosis. Arterioscler Thromb Vasc Biol 26(8):1729–1737
Heise M, Schmidmaier G, Husmann I, Heidenhain C, Schmidt J, Neuhaus P, Settmacher U (2006) PEG-hirudin/iloprost coating of small diameter ePTFE grafts effectively prevents pseudointima and intimal hyperplasia development. Eur J Vasc Endovasc Surg 32(4):418–424
Hirsh SL, McKenzie DR, Nosworthy NJ, Denman JA, Sezerman OU, Bilek MMM (2013) The vroman effect: competitive protein exchange with dynamic multilayer protein aggregates. Colloids Surf B Biointerfaces 103:395–404
Holme PA, Orvim U, Hamers M, Solum NO, Brosstad FR, Barstad RM, Sakariassen KS (1997) Shear-induced platelet activation and platelet microparticle formation at blood flow conditions as in arteries with a severe stenosis. Arterioscler Thromb Vasc Biol 17(4):646–653
Horbett TA (1993) Principles underlying the role of adsorbed plasma-proteins in blood interactions with foreign materials. Cardiovasc Pathol 2(3):137–148
John R, Lee S (2009) The biological basis of thrombosis and bleeding in patients with ventricular assist devices. J Cardiovasc Transl Res 2(1):63–70
Jones S, Evans RJ, Mahaut-Smith MP (2011) Extracellular Ca2+ modulates ADP-evoked aggregation through altered agonist degradation: implications for conditions used to study P2Y receptor activation. Br J Haematol 153(1):83–91
Jung F, Braune S, Lendlein A (2013) Haemocompatibility testing of biomaterials using human platelets. Clin Hemorheol Microcirc 53:97–115
Kador KE, Subramanian A (2011) Surface modification of biomedical grade polyurethane to enable the ordered co-immobilization of two proteins. J Biomater Sci Polym Ed 22(15):1983–1999
Keenan JP, Solum NO (1972) Quantitative studies on release of platelet fibrinogen by thrombin. Br J Haematol 23(4):461
Kragh T, Napoleone M, Fallah MA, Gritsch H, Schneider MF, Reininger AJ (2014) High shear dependent von Willebrand factor self-assembly fostered by platelet interaction and controlled by ADAMTS13. Thromb Res 133(6):1079–1087
Krajewski S, Krauss S, Kurz J, Neumann B, Schlensak C, Wendel HP (2014) Real-time measurement of free thrombin: evaluation of the usability of a new thrombin assay for coagulation monitoring during extracorporeal circulation. Thromb Res 133(3):455–463
Kuetting M, Roggenkamp J, Urban U, Schmitz-Rode T, Steinseifer U (2011) Polyurethane heart valves: past, present and future. Expert Rev Med Devices 8(2):227–233
Lam WA, Chaudhuri O, Crow A, Webster KD, Li T-D, Kita A, Huang J, Fletcher DA (2011) Mechanics and contraction dynamics of single platelets and implications for clot stiffening. Nat Mater 10(1):61–66
Lee D, Fong KP, King MR, Brass LF, Hammer DA (2012) Differential dynamics of platelet contact and spreading. Biophys J 102(3):472–482
Leiderman K, Fogelson AL (2011) Grow with the flow: a spatial-temporal model of platelet deposition and blood coagulation under flow. Math Med Biol 28(1):47–84
Leonard EF, Vroman L (1991) Is the Vroman effect of importance in the interaction of blood with artificial materials. J Biomater Sci Polym Ed 3(1):95–107
Li P, Cai X, Wang D, Chen S, Yuan J, Li L, Shen J (2013) Hemocompatibility and anti-biofouling property improvement of poly(ethylene terephthalate) via self-polymerization of dopamine and covalent graft of zwitterionic cysteine. Colloids Surf B Biointerfaces 110:327–332
Loscalzo J, Vaughan DE (1987) Tissue plasminogen-activator promotes platelet disaggregation in plasma. J Clin Investig 79(6):1749–1755
Modic M, Junkar I, Vesel A, Mozetic M (2012) Aging of plasma treated surfaces and their effects on platelet adhesion and activation. Surf Coat Technol 213:98–104
Morgenstern E, Korell U, Richter J (1984) Platelets and fibrin strands during clot retraction. Thromb Res 33(6):617–623
Moulin AM, O’Shea SJ, Badley RA, Doyle P, Welland ME (1999) Measuring surface-induced conformational changes in proteins. Langmuir 15(26):8776–8779
Neeves KB, Onasoga AA, Wufsus AR (2013) The use of microfluidics in hemostasis: clinical diagnostics and biomimetic models of vascular injury. Curr Opin Hematol 20(5):417–423
Ono A, Westein E, Hsiao S, Nesbitt WS, Hamilton JR, Schoenwaelder SM, Jackson SP (2008) Identification of a fibrin-independent platelet contractile mechanism regulating primary hemostasis and thrombus growth. Blood 112(1):90–99
Ratner BD (2007) The catastrophe revisited: blood compatibility in the 21st century. Biomaterials 28(34):5144–5147
Reininger AJ, Reininger CB, Wurzinger LJ (1993) The influence of fluid-dynamics upon adhesion of ADP-stimulated human platelets to endothelial-cells. Thromb Res 71(3):245–249
Reininger AJ, Korndorfer MA, Wurzinger LJ (1998) Adhesion of ADP-activated platelets to intact endothelium under stagnation point flow in vitro is mediated by the integrin alpha IIb beta 3. Thromb Haemost 79(5):998–1003
Reininger AJ, Agneskirchner J, Bode PA, Spannagl M, Wurzinger LJ (2000) C7E3 Fab inhibits low shear flow modulated platelet adhesion to endothelium and surface-adsorbed fibrinogen by blocking platelet GP IIb/IIIa as well as endothelial vitronectin receptor—results from patients with acute myocardial infarction and healthy controls. Thromb Haemost 83(2):217–223
Roest M, Reininger A, Zwaginga JJ, King MR, Heemskerk JWM (2011) Flow chamber-based assays to measure thrombus formation in vitro: requirements for standardization. J Thromb Haemost 9(11):2322–2324
Ruggeri ZM, Orje JN, Habermann R, Federici AB, Reininger AJ (2006) Activation-independent platelet adhesion and aggregation under elevated shear stress. Blood 108(6):1903–1910
Sadana A (1992) Protein adsorption and inactivation on surfaces—influence of heterogeneities. Chem Rev 92(8):1799–1818
Satulovsky J, Carignano MA, Szleifer I (2000) Kinetic and thermodynamic control of protein adsorption. Proc Nat Acad Sci USA 97(16):9037–9041
Schaller J, Kragh T, Reininger A, Goubergrits L, Kertzscher U, Spannagl M, Affeld K (2013) Biocompatibility material test for cardiovascular devices using stagnation point flow. Biomed Tech 58:3
Schonfeld F, Hessel V, Hofmann C (2004) An optimised split-and-recombine micro-mixer with uniform ‘chaotic’ mixing. Lab Chip 4(1):65–69
Shin IG, Kim DG, Kim HK, Kim SH, Jeon DM, Suh TS, Jang HS (2012) Development of drug eluting stent for the treatment of benign biliary stricture by electro-spray method. Polym Korea 36(2):163–168
Shu F, Parks R, Maholtz J, Ash S, Antaki JF (2009) Multimodal flow visualization and optimization of pneumatic blood pump for sorbent hemodialysis system. Artif Organs 33(4):334–345
Thakurta SG, Miller R, Subramanian A (2012) Adherence of platelets to in situ albumin-binding surfaces under flow conditions: role of surface-adsorbed albumin. Biomed Mater 7(4):1–10
Thoennissen NH, Schneider M, Allroggen A, Ritter MT, Dittrich R, Schmid C, Scheld HH, Ringelstein EB, Nabavi DG (2005) High level of cerebral microembolization in patients supported with the DeBakey left ventricular assist device. J Thorac Cardiovasc Surg 130(4):1159–1166
Trigwell S, De S, Sharma R, Mazumder MK, Mehta JL (2006) Structural evaluation of radially expandable cardiovascular stents encased in a polyurethane film. J Biomed Mater Res B Appl Biomater 76B(2):241–250
van Oeveren W, Tielliu IF, de Hart J (2012) Comparison of modified chandler, roller pump, and ball valve circulation models for in vitro testing in high blood flow conditions: application in thrombogenicity testing of different materials for vascular applications. Int J Biomater 2012:673163
Vermette P, Griesser H, Laroche G, Guidoin R (2001) Biomedical applications of polyurethanes. Landes Bioscience, Georgtown
Versteeg HH, Heemskerk JWM, Levi M, Reitsma PH (2013) New fundamentals in hemostasis. Physiol Rev 93(1):327–358
Vogler EA (2012) Protein adsorption in three dimensions. Biomaterials 33(5):1201–1237
Wellnhofer E, Osman J, Kertzscher U, Affeld K, Fleck E, Goubergrits L (2010) Flow simulation studies in coronary arteries—impact of side-branches. Atherosclerosis 213(2):475–481
Westein E, de Witt S, Lamers M, Cosemans JMEM, Heemskerk JWM (2012) Monitoring in vitro thrombus formation with novel microfluidic devices. Platelets 23(7):501–509
Zhang X, Halvorsen K, Zhang C-Z, Wong WP, Springer TA (2009) Mechanoenzymatic cleavage of the ultralarge vascular protein von Willebrand factor. Science 324(5932):1330–1334
Acknowledgments
The authors thank their colleagues from the thrombocyte transfusion service from the Department of Transfusion Medicine of the Medical Center of the University of Munich for their kind help with the blood collection. This work was supported by the German Research Foundation (DFG) [Grant Number GZ: RE 1293/5—1].
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 2 (MP4 8391 kb)
Supplementary material 3 (MP4 6561 kb)
Supplementary material 4 (MP4 5493 kb)
Supplementary material 5 (MP4 5727 kb)
Rights and permissions
About this article
Cite this article
Kragh, T., Schaller, J., Kertzscher, U. et al. Platelet adhesion, aggregation, and embolism on artificial surfaces in non-parallel blood flow. Microfluid Nanofluid 19, 155–167 (2015). https://doi.org/10.1007/s10404-015-1557-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10404-015-1557-5