Abstract
Teprotumumab (TPT) is a type I insulin-like growth factor receptor inhibitor, marketed as Tepezza; recently USFDA approved it for the treatment of thyroid eye disease (thyroid-associated ophthalmopathy (TAO), Graves ophthalmopathy/orbitopathy) in the USA. It is a monoclonal antibody although it was initially developed in collaboration with Genmab and Roche for the treatment of the tumour, but later it was investigated by River Vision Development Corporation and Horizon Therapeutics for its ophthalmic use. The drug has been designated as an orphan drug, breakthrough designation and fast-track designation. This review summarizes the milestones in the research and development including ongoing, clinical trial of TPT till now, foremost to this primary approval for thyroid-associated ophthalmopathy (TAO).

Similar content being viewed by others
Availability of data and material
The authors confirm that the data supporting the findings of this study are available within the article.
Abbreviations
- AUC:
-
Area under the curve
- AUCSS :
-
Area under the curve at steady state
- BLA:
-
Biologics license application
- C max :
-
Maximum serum concentration of the drug
- C min :
-
Minimum serum concentration of the drug
- C Trough :
-
Trough concentration
- CCL5:
-
Chemokine ligand 5
- DLT:
-
Dose-limiting toxicities
- DME:
-
Drug metabolizing enzymes
- GLP:
-
Good laboratory practices
- IGF-1:
-
Insulin-like growth factor 1
- IL-6/8:
-
Interleukin
- MTD:
-
Maximum tolerated dose
- OFs:
-
Orbital fibroblasts
- RES:
-
Reticuloendothelial system
- RVDC:
-
River Vision Development Corporation
- t 1/2 :
-
Half-life of the drug
- T max :
-
Time at which maximum serum concentration of the drug observed
- TED:
-
Thyroid eye disease
- TNF-α:
-
Tumour necrosis factor-α
- TSH:
-
Thyroid-stimulating hormone
- USFDA:
-
Food and Drug Administration
- V D :
-
Volume of distribution
- V SS :
-
Volume of distribution at steady state
References
Wang Y, Smith TJ (2014) Current concepts in the molecular pathogenesis of thyroid-associated ophthalmopathy. Invest Ophthalmol Vis Sci 55:1735–1748
Bahn RS (2010) Graves’ ophthalmopathy. N Engl J Med 362(8):726–738
Mellington FE, Dayan CM, Dickinson AJ et al (2017) Management of thyroid eye disease in the United Kingdom: a multicentre thyroid eye disease audit. Orbit 36:159–169
Hodgson NM, Rajaii F (2020) Current understanding of the progression and management of thyroid associated orbitopathy: a systematic review. Ophthalmol Ther 9:21–33. https://doi.org/10.1007/s40123-019-00226-9
Harris MA, Realini T, Hogg JP, Sivak-Callcott JA (2012) CT dimensions of the lacrimal gland in Graves orbitopathy. Ophthalmic Plast Reconstr Surg 28:69–72
Eckstein AK, Finkenrath A, Heiligenhaus A, Renzing-Köhler K, Esser J, Krüger C, Gieseler RK (2004) Dry eye syndrome in thyroid-associated ophthalmo-pathy: lacrimal expression of TSH receptor suggests involvement of TSHR-specific autoantibodies. Acta Ophthalmol Scand 82:291–297
Razek AAKA, Gaballa G, Denewer A, Tawakol I (2010) Diffusion weighted MR imaging of the breast. Acad Radiol 17:382–386
Razek AAKA, Abd E-G, Abdalla A, Fathy A, Azab A, Rahman AA (2009) Apparent diffusion coefficient vale of the brain in patients with Gaucher’s disease type II and type III. Neuroradiology 51:773
Razek AAKA, Elkhamary S, Mousa A (2011) Differentiation between benign and malignant orbital tumors at 3-T diffusion MR-imaging. Neuroradiology 53:517–522
Sepahdari AR, Politi LS, Aakalu VK, Kim HJ, Razek AA (2014) Diffusion-weighted imaging of orbital masses: multi-institutional data support a 2-ADC threshold model to categorize lesions as benign, malignant, or indeterminate. Am J Neuroradiol 35:170–175
Razek AAKA, Sadek AG, Gaballa G (2010) Diffusion-weighed MR of the thyroid gland in Graves’ disease: assessment of disease activity and prediction of outcome. Acad Radiol 17:779–783
Abdel Razek AAK, Allah SSA, El-Said AAEH (2017) Role of diffusion-weighted magnetic resonance (MR) imaging in differentiation between Graves’ disease and painless thyroiditis. Pol J Radiol 82:536–541
FDA approves first treatment for thyroid eye disease. FDA. https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-thyroid-eye-disease
Husted H, Relations I. Roche Presents positive preclinical data on roche presents positive preclinical data on. 51:1–2
2006 Annual Report Genmab is dedicated to creating and developing human antibodies to help people suffering from life-threatening and debilitating diseases. Our goal is to serve patients in need of new types of therapy and to build a business that maximi. 2006
Shan SJC, Douglas RS (2014) The pathophysiology of thyroid eye disease. J Neuro Ophthalmol 34(2):177–185
Heufelder AE, Smith TJ, Gorman CA, Bahn RS (1991) Increased Induction of HLA-DR by Interferon-γ in cultured fibroblasts derived from patients with graves’ Ophthalmopathy and pretibial dermopathy. J Clin Endocrinol Metab 73:307–313
Bartalena L, Wiersinga WM, Pinchera A (2004) Graves’ ophthalmopathy: state of the art and perspectives. J Endocrinol Invest 27:295–301
Smith TJ, Koumas L, Gagnon A, Bell A, Sempowski GD, Phipps RP et al (2002) Orbital fibroblast heterogeneity may determine the clinical presentation of thyroid-associated ophthalmopathy. J Clin Endocrinol Metab 87:385–392
Wiersinga WM (2011) Autoimmunity in Graves’ ophthalmopathy: the result of an unfortunate marriage between TSH receptors and IGF-1 receptors? J Clin Endocrinol Metab 96:2386–2694
Koumas L, Smith TJ, Phipps RP (2002) Fibroblast subsets in the human orbit: Thy-1+ and Thy-1- subpopulations exhibit distinct phenotypes. Eur J Immunol 32:477–485
Sorisky A, Pardasani D, Gagnon A, Smith TJ (1996) Evidence of adipocyte differentiation in human orbital fibroblasts in primary culture. J Clin Endocrinol Metab 81:3428–3431
Gillespie EF, Papageorgiou KI, Fernando R, Raychaudhuri N, Cockerham KP, Charara LK et al (2012) Increased expression of TSH receptor by fibrocytes in thyroid-associated ophthalmopathy leads to chemokine production. J Clin Endocrinol 97:E740–E746
Smith TJ, Bahn RS, Gorman CA, Cheavens M (1991) Stimulation of glycosaminoglycan accumulation by interferon gamma in cultured human retroocular fibroblasts. J Clin Endocrinol Metab 72:1169–1171
Smith TJ, Wang HS, Evans CH (1995) Leukoregulin is a potent inducer of hyaluronan synthesis in cultured human orbital fibroblasts. Am J Physiol Cell Physiol 268:C382–C388
Cao HJ, Wang HS, Zhang Y, Lin HY, Phipps RP, Smith TJ (1998) Activation of human orbital fibroblasts through CD40 engagement results in a dramatic induction of hyaluronan synthesis and prostaglandin endoperoxide H synthase-2 expression: insights into potential pathogenic mechanisms of thyroid-associated ophthalmopathy. J Biol Chem 273:29615–29625
Smith TJ, Hoa N (2004) Immunoglobulins from patients with graves’ disease induce hyaluronan synthesis in their orbital fibroblasts through the self-antigen, insulin-like growth factor-I receptor. J Clin Endocrinol Metab 89:5076–5080
Pritchard J, Han R, Horst N, Cruikshank WW, Smith TJ (2003) Immunoglobulin activation of T cell chemoattractant expression in fibroblasts from patients with Graves’ Disease is mediated through the insulin-like growth factor I receptor pathway. J Immunol 170:6348–6354
Pritchard J, Horst N, Cruikshank W, Smith TJ (2002) Igs from patients with Graves’ disease induce the expression of T cell chemoattractants in their fibroblasts. J Immunol 168:942–950
Douglas RS, Gianoukakis AG, Kamat S, Smith TJ (2007) Aberrant expression of the insulin-like growth factor-1 receptor by T cells from patients with Graves’ Disease may carry functional consequences for disease pathogenesis. J Immunol 178:3281–3287
Gianoukakis AG, Douglas RS, King CS, Cruikshank WW, Smith TJ (2006) Immunoglobulin G from patients with Graves’ disease induces interleukin-16 and RANTES expression in cultured human thyrocytes: a putative mechanism for T-cell infiltration of the thyroid in autoimmune disease. Endocrinology 147:1941–1949
Douglas RS (2019) Teprotumumab, an insulin-like growth factor-1 receptor antagonist antibody, in the treatment of active thyroid eye disease: a focus on proptosis. Eye 33:183–190
Markham A (2020) Teprotumumab: first approval. Drugs. https://doi.org/10.1007/s40265-020-01287-y
Chen H, Mester T, Raychaudhuri N, Kauh CY, Gupta S, Smith TJ, Douglas RS (2014) Teprotumumab, an IGF-1R blocking monoclonal antibody inhibits TSH and IGF-1 action in fibrocytes. J Clin Endocrinol Metab 99:E1635–E1640. https://doi.org/10.1210/jc.2014-1580
Kenakin TP (2019) Pharmacokinetics. In: a pharmacology primer. Elsevier. https://linkinghub.elsevier.com/retrieve/pii/B9780128139578000096
Flynn E (2007) Clinical PHARMACOKINETICS. In: xPharm: the comprehensive pharmacology reference. Elsevier Inc.. https://linkinghub.elsevier.com/retrieve/pii/B9780080552323600327
Kester M, Karpa KD, Vrana KE (2012) Pharmacokinetics. In: Elsevier’s integrated review pharmacology. Elsevier. https://linkinghub.elsevier.com/retrieve/pii/B978032307445200001X
Kurzrock R, Patnaik A, Aisner J, Warren T, Leong S, Benjamin R et al (2010) A phase I study of weekly R1507, a human monoclonal antibody insulin-like growth factor-I receptor antagonist, in patients with advanced solid tumors. Clin Cancer Res 16:2458–2465
Rodon J, Patnaik A, Stein M, Tolcher A, Ng C, Dias C et al (2007) A phase I study of q3W R1507, a human monoclonal antibody IGF-1R antagonist in patients with advanced cancer. J Clin Oncol 25:3590–3590
Fda. Center for drug evaluation and research application number: 761143orig1s000 Clinical Review(S)
Fda. Center for drug evaluation and research
Tabrizi MA, Tseng CML, Roskos LK (2006) Elimination mechanisms of therapeutic monoclonal antibodies. Drug Discov Today 11:81–88
Wang W, Wang EQ, Balthasar JP (2008) Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther 84:548–558
Bagatell R, Herzog CE, Trippett TM, Grippo JF, Cirrincione-Dall G, Fox E et al (2011) Pharmacokinetically guided phase 1 trial of the IGF-1 receptor antagonist RG1507 in children with recurrent or refractory solid tumors. Clin Cancer Res 17:611–619
Balis FM, Fox E (2012) Challenges of developing new drugs for childhood cancers. Clin Invest 2:291–300
Nagayama Y, Nakahara M, Abiru N (2015) Animal models of Graves’ disease and Graves’ orbitopathy. Curr Opin Endocrinol Diabetes Obes 22(5):381–386. https://doi.org/10.1097/MED.0000000000000186
Ungerer M, Fabender J, Li Z, Münch G, Holthoff HP (2017) Review of mouse models of Graves’ disease and orbitopathy—novel treatment by induction of tolerance. Clin Rev Allergy Immunol 52(2):182–193. https://doi.org/10.1007/s12016-016-8562-7
Fda, Cder. Highlights of prescribing information [Internet]. www.fda.gov/medwatch
Douglas RS, Kahaly GJ, Patel A, Sile S, Thompson EHZ, Perdok R et al (2020) Teprotumumab for the treatment of active thyroid eye disease. N Engl J Med 382:341–352
A study of R1507 in combination with multiple standard chemotherapy treatments in patients with advanced solid tumors: full text view. https://clinicaltrials.gov/ct2/show/NCT00811993?term=NO22068&draw=2&rank=1
Pappo AS, Vassal G, Crowley JJ, Bolejack V, Hogendoorn PCW, Chugh R et al (2014) A phase 2 trial of R1507, a monoclonal antibody to the insulin-like growth factor-1 receptor (IGF-1R), in patients with recurrent or refractory rhabdomyosarcoma, osteosarcoma, synovial sarcoma, and other soft tissue sarcomas: results of a Sarcoma Alliance for research through collaboration study. Cancer 120:2448–2456
A study of R1507 in combination with letrozole in postmenopausal women with advanced breast cancer: full text view. https://clinicaltrials.gov/ct2/show/NCT00796107?term=RG1507&draw=2&rank=4
A study of the effect of R1507 in combination with tarceva (Erlotinib) on progression-free survival in patients with stage IIIb/IV non-small cell lung cancer (NSCLC) having received tarceva monotherapy: full text view. https://clinicaltrials.gov/ct2/show/NCT00773383?term=RG1507&draw=2&rank=5
Cunningham C, Zendel L. (2019) Center for drug evaluation and research application number: 761143Orig1s000 risk assessment and risk mitigation review(S) 1 division of risk management (DRM) office of medication error prevention and risk management (OMEPRM) Office of Surveillance and Epidemiology (OSE) center for Drug evaluation and research (CDER) application type BLA application number 761143 PDUFA Goal date subject evaluation of need for a REMS. 2019
Smith TJ, Kahaly GJ, Ezra DG et al (2017) Teprotumumab for thyroid-associated ophthalmopathy. N Engl J Med 376:1748–1761
Teprotumumab (RV 001) treatment in patients with active thyroid eye disease: full text view. https://clinicaltrials.gov/ct2/show/NCT01868997?term=teprotumumab&draw=2&rank=5
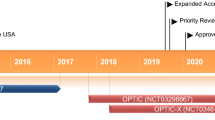
Treatment of Graves’ orbitopathy (thyroid eye disease) to reduce proptosis with teprotumumab infusions in a randomized, placebo-controlled, clinical study: full text view. https://clinicaltrials.gov/ct2/show/NCT03298867?term=teprotumumab&draw=2&rank=2
Stan MN, Garrity JA, Carranza Leon BG et al (2015) Randomized controlled trial of rituximab in patients with Graves’ orbitopathy. J Clin Endocrinol Metab 100:432–441
Goldman JW, Mendenhall MA, Rettinger SR (2016) Hyperglycemia associated with targeted oncologic treatment: mechanisms and management. Oncologist 21:1326–1336
Weiler DL (2017) Thyroid eye disease: a review. Clin Exp Optom 100:20–25
Smith TJ, Hegedüs L (2016) Graves’ disease. N Engl J Med 375:1552–1565
Barrio-Barrio J, Sabater AL, Bonet-Farriol E, Velázquez-Villoria Á, Galofré JC (2015) Graves’ ophthalmopathy: VISA versus EUGOGO classification, assessment, and management. J Ophthalmol. https://doi.org/10.1155/2015/249125
Byun JS, Moon NJ, Lee JK (2017) Quantitative analysis of orbital soft tissues on computed tomography to assess the activity of thyroid-associated orbitopathy. Graefe’s Arch Clin Exp Ophthalmol 255:413–420
Huh HD, Kim JH, Kim SJ, Yoo JM, Seo SW (2016) The change of lacrimal gland volume in Korean patients with thyroid-associated ophthalmopathy. Korean J Ophthalmol 30:319–325
Hu H, Xu XQ, Wu FY, Chen HH, Su GY, Shen J, Shi HB (2016) Diagnosis and stage of Graves’ ophthalmopathy: efficacy of quantitative measurements of the lacrimal gland based on 3-T magnetic resonance imaging. Exp Ther Med 12:725–729
Bingham CM, Harris MA, Realini T, Nguyen J, Hogg JP, Sivak-Callcott JA (2014) Calculated computed tomography volumes of lacrimal glands and comparison to clinical findings in patients with thyroid eye disease. Ophthalmic Plast Reconstr Surg 30:116–118
Xu XQ, Hu H, Su GY, Liu H, Shi HB, Wu FY (2016) Diffusion weighted imaging for differentiating benign from malignant orbital tumors: diagnostic performance of the apparent diffusion coefficient based on region of interest selection method. Korean J Radiol 17:650–656
A phase 1, Open-label study of teprotumumab in patients with diabetic macular edema (DME): full text view. https://clinicaltrials.gov/ct2/show/NCT02103283?term=teprotumumab&draw=2&rank=4
Expanded access protocol of teprotumumab (HZN-001) for patients with active thyroid eye disease: full text view. https://clinicaltrials.gov/ct2/show/NCT04040894?term=teprotumumab&draw=2&rank=3
Treatment of Graves’ orbitopathy to reduce proptosis with teprotumumab infusions in an open-label clinical extension study: full text view. https://clinicaltrials.gov/ct2/show/NCT03461211?term=teprotumumab&draw=2&rank=1
A study of R1507 in participants with recurrent or refractory sarcoma: full text view. https://clinicaltrials.gov/ct2/show/NCT00642941?term=NO21157&draw=2&rank=1
Asmane I, Watkin E, Alberti L, Duc A, Marec-Berard P, Ray-Coquard I et al (2012) Insulin-like growth factor type 1 receptor (IGF-1R) exclusive nuclear staining: a predictive biomarker for IGF-1R monoclonal antibody (Ab) therapy in sarcomas. Eur J Cancer 48:3027–3035
Pappo AS, Patel SR, Crowley J, Reinke DK, Kuenkele KP, Chawla SP et al (2011) R1507, a monoclonal antibody to the insulin-like growth factor 1 receptor, in patients with recurrent or refractory Ewing sarcoma family of tumors: results of a phase II Sarcoma Alliance for research through Collaboration study. J Clin Oncol 29:4541–4547
A multiple ascending dose study of R1507 in children and adolescents with advanced solid tumors: full text view. https://clinicaltrials.gov/ct2/show/NCT00560144?term=NO21200&draw=2&rank=1
A multiple ascending dose study of R1507 in patients with advanced solid tumors: tabular view. https://clinicaltrials.gov/ct2/show/record/NCT00400361?term=BO19373&draw=2&rank=1
Mahadevan D, Sutton GR, Arteta-Bulos R, Bowden CJ, Miller PJE, Swart RE et al (2014) Phase 1b study of safety, tolerability and efficacy of R1507, a monoclonal antibody to IGF-1R in combination with multiple standard oncology regimens in patients with advanced solid malignancies. Cancer Chemother Pharmacol 73:467–473
A study to evaluate the biological activity of R1507 in women with operable breast cancer: full text view. https://clinicaltrials.gov/ct2/show/NCT00882674?term=RG1507&draw=2&rank=1
A multiple ascending dose study of the mTOR inhibitor (RAD001) in combination with R1507 in patients with advanced solid tumors: full text view. https://clinicaltrials.gov/ct2/show/NCT00985374?term=RG1507&draw=2&rank=6
Ramalingam SS, Spigel DR, Chen D, Steins MB, Engelman JA, Schneider CP et al (2011) Randomized phase II study of erlotinib in combination with placebo or R1507, a monoclonal antibody to insulin-like growth factor-1 receptor, for advanced-stage non-small-cell lung cancer. J Clin Oncol 29:4574–4580
A Study of the effect of R1507 in combination with Tarceva (Erlotinib) on progression-free survival in patients with stage IIIb/IV non-small cell lung cancer (NSCLC). Full Text View. https://clinicaltrials.gov/ct2/show/NCT00760929?term=RG1507&draw=2&rank=8
Acknowledgements
The authors wish to express their gratitude to Botswana Medicines Regulatory Authority, Gaborone, Botswana.
Funding
This project is not funded by any grant or agency.
Author information
Authors and Affiliations
Contributions
FA and VA contributed to the conception and design. The first draft of the manuscript was written by AC, and all authors commented on previous versions of the manuscript. All authors read, approved and permitted the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ali, F., Chorsiya, A., Anjum, V. et al. Teprotumumab (Tepezza): from the discovery and development of medicines to USFDA approval for active thyroid eye disease (TED) treatment. Int Ophthalmol 41, 1549–1561 (2021). https://doi.org/10.1007/s10792-021-01706-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-021-01706-3