Abstract
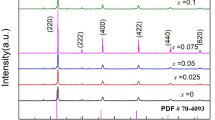
La0.6Sr0.4Co0.2Fe0.8O3−δ (LSCF), La0.6Sr0.4Co0.2Fe0.78Mo0.02O3−δ (LSCFM02), La0.6Sr0.4Co0.2Fe0.75Mo0.05O3−δ (LSCFM05) cathodes were prepared and their electrochemical performance and stability were investigated. Mo doping into LSCF, which is confirmed by X-ray diffraction (XRD) and Rietveld refinement, increases unit cell parameters from 3.893 to 3.924 Å, causing expansion of unit cell volume. Polarization resistance (Rp) value of LSCFM05 cathodes is less that of LSCF cathodes at 750 °C, indicating that Mo-doped LSCF exhibits enhanced electrochemical performance. X-ray photoelectron spectroscopy (XPS) analysis shows that high electrocatalytic activity for oxygen reduction reaction of Mo-doped LSCF cathodes is related to mixed-valent Mo5+/Mo6+. LSCFM05 cathodes have less degradation rate during 20 h testing at 700 °C in air compared to LSCF cathodes. XPS results show that Mo doping reduces Sr surface segregation and is responsible for the stability enhancement of LSCF cathodes.
Graphic Abstract








Similar content being viewed by others
References
Wang S, Jiang SP (2017) Prospects of fuel cell technologies. Natl Sci Rev 4(2):163–166
Stambouli AB, Traversa E (2002) Solid oxide fuel cells (SOFCs): a review of an environmentally clean and efficient source of energy. Renew Sust Energ Rev 6(5):433–455
Steele BCH (2001) Material science and engineering: the enabling technology for the commercialisation of fuel cell systems. J Mater Sci 36(5):1053–1068
Yokokawa H, Tu H, Iwanschitz B, Mai A (2008) Fundamental mechanisms limiting solid oxide fuel cell durability. J Power Sources 182(2):400–412
Brett DJ, Atkinson A, Brandon NP, Skinner SJ (2008) Intermediate temperature solid oxide fuel cells. Chem Soc Rev 37(8):1568–1578
Cao Y, Gadre MJ, Ngo AT, Adler SB, Morgan DD (2019) Factors controlling surface oxygen exchange in oxides. Nat Commun 10(1):1346
Abdalla AM, Hossain S, Azad AT, Petra PMI, Begum F, Eriksson SG, Azad AK (2018) Nanomaterials for solid oxide fuel cells: a review. Renew Sust Energ Rev 82:353–368
Chen Y, Zhang L, Wang C, Cai H, Wang L, Song Z (2018) Performance of La0.5Sr0.5Fe0.9Mo0.1O3−δ–Sm0.2Ce0.8O2−δ composite cathode for CeO2- and LaGaO3-based solid oxide fuel cells. Ionics 24(9):2717–2728
Tai LW, Nasrallah MM, Anderson HU, Sparlin DM, Sehlin SR (1995) Structure and electrical properties of La1-xSrxCo1-yFeyO3. Part 1. The system La0.8Sr0.2Co1-yFeyO3. Solid State Ionics 76:259–271
Jiang SP (2002) A comparison of O2 reduction reactions on porous (La, Sr)MnO3 and (La, Sr)(Co, Fe)O3 electrodes. Solid State Ionics 146(1–2):1–22
Giuliano A, Carpanese MP, Clematis D, Boaro M, Pappacena A, Deganello F, Liotta LF, Barbucci A (2017) Infiltration, overpotential and ageing effects on cathodes for solid oxide fuel cells: La0.6Sr0.4Co0.2Fe0.8O3-δ versus Ba0.5Sr0.5Co0.8Fe0.2O3-δ. J Electrochem Soc 164(10):F3114–F3122
Song YH, Rehman SU, Kim HS, Song HS, Song RH, Lim TH, Hong JE, Park SJ, Huh JY, Lee SB (2020) Facile surface modification of LSCF/GDC cathodes by epitaxial deposition of Sm0.5Sr0.5CoO3 via ultrasonic spray infiltration. J Mater Chem A 8(7):3967–3977
Simner SP, Anderson MD, Engelhard MH, Stevenson JW (2006) Degradation mechanisms of La-Sr-Co-Fe-O3 SOFC cathodes. Electrochem Solid State Lett 9(10):A478–A481
Chen K, Li N, Ai N, Cheng Y, Rickard WD, Jiang SP (2016) Polarization-Induced interface and Sr segregation of in situ assembled La0.6Sr0.4Co0.2Fe0.8O3-δ electrodes on Y2O3-ZrO2 electrolyte of solid oxide fuel cells. ACS Appl Mater Interfaces 8(46):31729–31737
Lu Z, Darvish S, Hardy J, Templeton J, Stevenson J, Zhong Y (2017) SrZrO3 formation at the interlayer/electrolyte interface during (La1-xSrx)1-δCo1-yFeyO3 cathode sintering. J Electrochem Soc 164(10):F3097–F3103
Liu Y, Zhao X, Yang Z, Wang Z, Chen X, Yang S, Wei M (2019) New insights into element migration on La0.6Sr0.4Co0.2Fe0.8O3-δ cathodes of intermediate temperature solid oxide fuel cells. Solid State Ionics 334:145–151
Wang GY, Zhang YL, Han MF (2020) Densification of Ce0.9Gd0.1O2-δinterlayer to improve the stability of La0.6Sr0.4Co0.2Fe0.8O3-δ/Ce0.9Gd0.1O2-δ interface and SOFC. J Electroanal Chem 857:113591
Chen K, He S, Li N, Cheng Y, Ai N, Chen M, Rickard WDA, Zhang T, Jiang SP (2018) Nb and Pd co-doped La0.57Sr0.38Co0.19Fe0.665Nb0.095Pd0.05O3-δ as a stable, high performance electrode for barrier-layer-free Y2O3-ZrO2 electrolyte of solid oxide fuel cells. J Power Sources 378:433–442
Yoo S, Kim J, Song SY, Lee DW, Shin J, Ok KM, Kim G (2014) Structural, electrical and electrochemical characteristics of La0.1Sr0.9Co1−xNbxO3−δ as a cathode material for intermediate temperature solid oxide fuel cells. RSC Adv 4(36):18710–18717
Wang J, Yang T, Lei L, Huang K (2017) Ta-Doped SrCoO3−δ as a promising bifunctional oxygen electrode for reversible solid oxide fuel cells: a focused study on stability. J Mater Chem A 5(19):8989–9002
He W, Wu X, Yang G, Shi H, Dong F, Ni M (2017) BaCo0.7Fe0.22Y0.08O3−δ as an active oxygen reduction electrocatalyst for low-temperature solid oxide fuel cells below 600 °C. ACS Energy Lett 2(2):301–305
Meffert M, Müller P, Störmer H, Unger LS, Niedrig C, Wagner SF, Saher S, Bouwmeester H, Ivers-Tiffée E, Gerthsen D (2014) Effect of yttrium (Y) and zirconium (Zr) doping on the thermodynamical stability of the cubic Ba0.5Sr0.5Co0.8Fe0.2O3-δ phase. Microsc Microanal 20(S3):466–467
Zhou F, Liu Y, Zhao X, Tang W, Yang S, Zhong S, Wei M (2018) Effects of cerium doping on the performance of LSCF cathodes for intermediate temperature solid oxide fuel cells. Int J Hydrog Energy 43(41):18946–18954
Park BK, Barnett SA (2020) Boosting solid oxide fuel cell performanceviaelectrolyte thickness reduction and cathode infiltration. J Mater Chem A 8(23):11626–11631
Niu B, Jin F, Zhang L, Shen P, He T (2018) Performance of double perovskite symmetrical electrode materials Sr2TiFe1–xMoxO6–δ ( x = 0.1, 0.2) for solid oxide fuel cells. Electrochim Acta 263:217–227
Zhang P, Guan G, Khaerudini DS, Hao X, Xue C, Han M, Kasai Y, Abudula A (2015) B-site Mo-doped perovskite Pr0.4Sr0.6(Co0.2Fe0.8)1−xMoxO3−σ ( x = 0, 0.05, 0.1 and 0.2) as electrode for symmetrical solid oxide fuel cell. J Power Sources 276:347–356
Xiao G, Liu Q, Wang S, Komvokis VG, Amiridis MD, Heyden A, Ma S, Chen F (2012) Synthesis and characterization of Mo-doped SrFeO3−δ as cathode materials for solid oxide fuel cells. J Power Sources 202:63–69
Adler SB (2004) Factors governing oxygen reduction in solid oxide fuel cell cathodes. Chem Rev 104(10):4791–4843
Liu B, Zhang Y, Zhang L (2008) Characteristics of Ba0.5Sr0.5Co0.8Fe0.2O3−δ–La0.9Sr0.1Ga0.8Mg0.2O3−δ composite cathode for solid oxide fuel cell. J Power Sources 175(1):189–195
Serra JM, Buchkremer HP (2007) On the nanostructuring and catalytic promotion of intermediate temperature solid oxide fuel cell (IT-SOFC) cathodes. J Power Sources 172(2):768–774
Chen J, Liang FL, Chi B, Pu J, Jiang SP, Li J (2009) Palladium and ceria infiltrated La0.8Sr0.2Co0.5Fe0.5O3-δ cathodes of solid oxide fuel cells. J Power Sources 194(1):275–280
Kumar P, Presto S, Sinha ASK, Varma S, Viviani M, Singh P (2017) Effect of samarium (Sm3+) doping on structure and electrical conductivity of double perovskite Sr2NiMoO6 as anode material for SOFC. J Alloy Compd 725:1123–1129
Zhen SY, Sun W, Tang GZ, Rooney D, Sun KN, Ma XX (2016) Evaluation of strontium-site-deficient Sr2Fe1.4Co0.1Mo0.5O6-δ-based perovskite oxides as intermediate temperature solid oxide fuel cell cathodes. Int J Hydrog Energy 41(22):9538–9546
Liu Y, Chen K, Zhao L, Chi B, Pu J, Jiang SP, Jian L (2014) Performance stability and degradation mechanism of La0.6Sr0.4Co0.2Fe0.8O3−δ cathodes under solid oxide fuel cells operation conditions. Int J Hydrog Energy 39(28):15868–15876
van der Heide PAW (2002) Systematic x-ray photoelectron spectroscopic study of La1-xSrx-based perovskite-type oxides. Surf Interface Anal 33(5):414–425
Chen K, Hyodo J, Ai N, Ishihara T, Jiang SP (2016) Boron deposition and poisoning of La0.8Sr0.2MnO3 oxygen electrodes of solid oxide electrolysis cells under accelerated operation conditions. Int J Hydrog Energy 41(3):1419–1431
Acknowledgements
The project was supported by Open Foundation of Guangxi Key Laboratory of Processing for Non-ferrous Metals and Featured Materials, Guangxi University (2019GXYSOF11), Hubei Superior and Distinctive Discipline Group of “Mechatronics and Automobiles” (XKQ2019060) and the 111 Project (B17034). XRD, SEM and TG examinations were assisted by the Center of Material Research and Analysis of Wuhan University of Technology.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, Y., Zhou, F., Chen, X. et al. Enhanced electrochemical activity and stability of LSCF cathodes by Mo doping for intermediate temperature solid oxide fuel cells. J Appl Electrochem 51, 425–433 (2021). https://doi.org/10.1007/s10800-020-01515-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-020-01515-z