Abstract
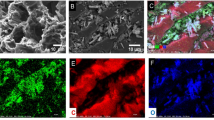
A three-dimensional (3D) activated framework carbon (3D AFC) was prepared in our research group and applied to adsorb U(VI) from radioactive waste water. Meanwhile, The effects of solid–liquid ratio, contact time, pH value, ionic strength and ionic concentration of adsorbate on the adsorption of U(VI) by the 3D AFC were studied. The calculated maximum adsorption capacity of 3D AFC was 127.5 mg/g under the condition that pH was 5.8. Combined with the properties of excellent three-dimensional network structure, simple synthesis methodology, and the adsorption capacity for U(VI), 3D AFC is considered to be feasible for recycling U(VI) from waste water solutions.








Similar content being viewed by others
References
Brook BW, Alonso A, Meneley DA, Misak J, Blees T, van Erp JB (2014) Why nuclear energy is sustainable and has to be part of the energy mix. SM&T 1–2:8–16
Gavrilescu M, Pavel LV, Cretescu I (2009) Characterization and remediation of soils contaminated with uranium. J Hazard Mater 163:475–510
Schnug E, Lottermoser BG (2013) Fertilizer-derived uranium and its threat to human health. Environ Sci Technol 47:2433–2434
Olatunji MA, Khandaker MU, Mahmud HNME, Amin YM (2015) Influence of adsorption parameters on cesium uptake from aqueous solutions- a brief review. RSC Adv 5:71658–71683
Kameda T, Suzuki Y, Yoshioka T (2014) Removal of arsenic from an aqueous solution by coprecipitation with manganese oxide. J Environ Chem Eng 2:2045–2049
Tag El-Din AF, El-Khouly ME, Elshehy EA, Atia AA, El-Said WA (2018) Cellulose acetate assisted synthesis of worm-shaped mesopores of MgP ion-exchanger for cesium ions removal from seawater. Micropor Mesopor Mat 265:211–218
Sunil K, Karunakaran G, Yadav S, Padaki M, Zadorozhnyy V, Pai RK (2018) Al-Ti2O6 a mixed metal oxide based composite membrane: a unique membrane for removal of heavy metals. Chem Eng J 348:678–684
Khorzughy SH, Eslamkish T, Ardejani FD, Heydartaemeh MR (2014) Cadmium removal from aqueous solutions by pumice and nano-pumice. Korean J Chem Eng 32:88–96
Yakout SM, Rizk MA (2013) Adsorption of uranium by low-cost adsorbent derived from agricultural wastes in multi-component system. Desalin Water Treat 53:1917–1922
Yakout SM, Metwally SS, El-Zakla T (2013) Uranium sorption onto activated carbon prepared from rice straw: competition with humic acids. Appl Surf Sci 280:745–750
Kütahyalı C, Eral M (2010) Sorption studies of uranium and thorium on activated carbon prepared from olive stones: kinetic and thermodynamic aspects. J Nucl Mater 396:251–256
Lv Z, Wang H, Chen C, Yang S, Chen L, Alsaedi A, Hayat T (2019) Enhanced removal of uranium(VI) from aqueous solution by a novel Mg-MOF-74-derived porous MgO/carbon adsorbent. J Colloid Interf Sci 537:A1–A10
Abney CW, Mayes RT, Saito T, Dai S (2017) Materials for the recovery of uranium from seawater. Chem Rev 117:13935–14013
Mellah A, Chegrouche S, Barkat M (2006) The removal of uranium(VI) from aqueous solutions onto activated carbon: Kinetic and thermodynamic investigations. J Colloid Interf Sci 296:434–441
Bhatnagar A, Hogland W, Marques M, Sillanpää M (2013) An overview of the modification methods of activated carbon for its water treatment applications. Chem Eng J 219:499–511
Zhao Y, Liu C, Feng M, Chen Z, Li S, Tian G, Wang L, Huang J, Li S (2010) Solid phase extraction of uranium(VI) onto benzoylthiourea-anchored activated carbon. J Hazard Mater 176:119–124
Vivero-Escoto JL, Carboni M, Abney CW, deKrafft KE, Lin W (2013) Organo-functionalized mesoporous silicas for efficient uranium extraction. Micropor Mesopor Mat 180:22–31
Wang H, Ma L, Cao K, Geng J, Liu J, Song Q, Yang X, Li S (2012) Selective solid-phase extraction of uranium by salicylideneimine-functionalized hydrothermal carbon. J Hazard Mater 229–230:321–330
Li H, Li Y, Zhou Y, Li B, Liu D, Liao H (2019) Efficient removal of uranium using a melamine/trimesic acid-modified hydrothermal carbon-based supramolecular organic framework. J Colloid Interf Sci 544:14–24
Yang B, Chen J, Lei S, Guo R, Li H, Shi S, Yan X (2018) Spontaneous growth of 3D framework carbon from sodium citrate for high energy- and power-density and long-life sodium-ion hybrid capacitors. Adv Energy Mater 8:1702409
Cao L, Fan F (2020) Deformation and instability of three-dimensional graphene honeycombs under in-plane compression: atomistic simulations. Extreme Mech Lett 39:100861
Zhang Y, Guan W, Wang Q, Wang X, Lai X, Shuai M (2010) Study the oxidation kinetics of uranium using XRD and Rietveld method. IOP Conference Series: Mater Sci Eng 9:012019
Compton OC, Nguyen ST (2010) Graphene oxide, highly reduced graphene oxide, and graphene: versatile building blocks for carbon-based materials. SMALL 6:711–723
Ai Y, Liu Y, Lan W, Jin J, Xing J, Zou Y, Zhao C, Wang X (2018) The effect of pH on the U(VI) sorption on graphene oxide (GO): a theoretical study. Chem Eng J 343:460–466
Sajjad S, Khan Leghari SA, Iqbal A (2017) Study of graphene oxide structural features for catalytic, antibacterial, gas sensing, and metals decontamination environmental applications. ACS Appl Mater Interfaces 9:43393–43414
Gao H, Sun Y, Zhou J, Xu R, Duan H (2013) Mussel-inspired synthesis of polydopamine-functionalized graphene hydrogel as reusable adsorbents for water purification. ACS Appl Mater Interfaces 5:425–432
Xie Y, Chen C, Ren X, Wang X, Wang H, Wang X (2019) Emerging natural and tailored materials for uranium-contaminated water treatment and environmental remediation. Prog Mater Sci 103:180–234
Chen L, Lu S (2008) Sorption and desorption of radiocobalt on montmorillonite-effects of pH, ionic strength and fulvic acid. Appl Radiat Isot 66:288–294
Zhou W, Wang J, He J, Yang X, Shi Y, Wang X, Liu C (2019) Adsorption of U(VI) on montmorillonite in the presence of ethylenediaminetetraacetic acid. Colloids Sur A 583:123929
Kilislioglu A (2003) The effect of various cations and pH on the adsorption of U(VI) on Amberlite IR-118H resin. Appl Radiat Isot 58:713–717
Okeola FO, Odebunmi EO (2010) Comparison of Freundlich and Langmuir isotherms for adsorption of methylene blue by agrowaste derived activated carbon. Adv Environ Biol 4:329–335
Acknowledgements
We thank the Natural Science Foundation of China (20190431), the Clinical Research Foundation of Western Stomatology of Chinese Dental Association (CSA-W2018-07).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Song, F., Zhang, Z., Mai, X. et al. Efficient removal of U(VI) from aqueous solutions via an activated 3D framework carbon. J Radioanal Nucl Chem 327, 721–729 (2021). https://doi.org/10.1007/s10967-020-07541-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-020-07541-7