Abstract
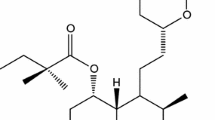
This paper deals with the study of compatibility between antihyperlipidemic agent atorvastatin calcium trihydrate (ATV) and eight pharmaceutical excipients used in the development of solid dosage forms, namely citric acid, anhydrous lactose, magnesium citrate, magnesium carbonate, sodium carboxymethyl cellulose, polyvinylpyrrolidone K30, colloidal silica and sorbitol. As investigational tools, universal attenuated total reflectance Fourier transform infrared spectroscopy and powder X-ray diffractogram patterns were used for binary mixtures of ATV with each excipient at ambient condition and then completed by subjecting the samples to thermal stress using thermal analysis (TG/DTG/HF), in non-isothermal conditions and in oxidative medium. It was shown the binary mixtures do not present interactions between ATV and excipients when stored under ambient conditions for 2 months, while under thermal stress, ATV presents interactions with sorbitol.






Similar content being viewed by others
References
Chadha R, Bhandari S. Drug-excipient compatibility screening–role of thermoanalytical and spectroscopic techniques. J Pharmaceut Biomed Anal. 2014;87:82–97.
Liltorp K, Larsen TG, Willumsen B, Holm R. Solid state compatibility studies with tablet excipients using non thermal methods. J Pharm Biomed Anal. 2011;55(3):424–8.
Bhattacharyya L, Schuber S, Sheeha C, William R. Excipients: Background/Introduction. In: Katdare A, Chaubal M, editors. Excipient development for pharmaceutical, biotechnology, and drug delivery systems. New York: Informa Healthcare USA, Inc.; 2006. p. 1–2.
Bharate SS, Bharate SB, Bajaj AN. Interactions and incompatibilities of pharmaceutical excipients with active pharmaceutical ingredients: a comprehensive review. J Excip Food Chem. 2010;1(3):3–26.
Julio AT, Zamara IF, Garcia JS, Trevisan MG. Compatibility of sildenafil citrate and pharmaceutical excipients by thermal analysis and LC–UV. J Therm Anal Calorim. 2013;111:2037–44.
Bruni G, Berbenni V, Milanese C, Girella A, Marini A. Drug-excipient compatibility studies in binary and ternary mixtures by physico-chemical techniques. J Therm Anal Calorim. 2010;102:193–201.
de Barros Lima IP, Lima NGPB, Barros DMC, et al. Compatibility study between hydroquinone and the excipients used in semi-solid pharmaceutical forms by thermal and non-thermal techniques. J Therm Anal Calorim. 2015;120:719–32.
Stanisz B, Regulska K, Kania J, Garbacki P. Effect of pharmaceutical excipients on the stability of angiotensin-converting enzyme inhibitors in their solid dosage formulations. Drug Dev Ind Pharm. 2013;39(1):51–61.
Djordjevic FN, Antonijevic MD, Pavlovic A, Vuckovic I, Nikolic K, Agbaba D. The stress stability of olanzapine: studies of interactions with excipients in solid state pharmaceutical formulations. Drug Dev Ind Pharm. 2015;41(3):502–14.
Daniel JSP, Veronez IP, Rodrigues LL, Trevisan MG, Garcia JS. Risperidone–Solid-state characterization and pharmaceutical compatibility using thermal and non-thermal techniques. Thermochim Acta. 2013;568:148–55.
Ledeti A, Vlase G, Vlase T, et al. Solid-state preformulation studies of amiodarone hydrochloride. J Therm Anal Calorim. 2016;126(1):181–7.
Buda V, Andor M, Ledeti A, Ledeti I, et al. Comparative solid-state stability of perindopril active substance vs. pharmaceutical formulation. Int J Mol Sci. 2017;18:164. https://doi.org/10.3390/ijms18010164.
Fulias, et al. Thermal behaviour of procaine and benzocaine Part II: compatibility study with some pharmaceutical excipients used in solid dosage forms. Chem Cent J. 2013;7:140.
Monkhouse DC, Maderich A. Whither compatibility testing? Drug Dev Ind Pharm. 1989;15:2115–30.
http://www.drugbank.ca/drugs/DB01076. Accessed 26 Feb 2017.
https://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/UnderstandingGenericDrugs/ucm167991.htm. Accessed 25 Feb 2017.
http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020702s057lbl. Accessed 22 Feb 2017.
Kerc J, Salobir M, Bavec B. 2006; Patent US 7030151 B2.
https://pubchem.ncbi.nlm.nih.gov/compound/atorvastatin. Accessed 23 Feb 2017.
Ledeti I, Vlase G, Vlase T, Suta LM, Todea A, Fulias A. Selection of solid-state excipients for simvastatin dosage forms through thermal and nonthermal techniques. J Therm Anal Calorim. 2015;121(3):1093–102.
Ledeti I, Vlase G, Vlase T, Ciucanu I, Olariu T, Todea A, Fulias A, Suta LM. Instrumental analysis of potential lovastatin—excipient interactions in preformulation studies. Rev Chim. 2015;66(6):879–82.
da Silva EP, Pereira MAV, de Barros Lima IP, et al. Compatibility study between atorvastatin and excipients using DSC and FTIR. J Therm Anal Calorim. 2016;123:933–9.
Dewan I, Shahriar M, Islam SMA. Study of differential scanning calorimetry of atorvastatin in solid solution. Bangladesh Pharm J. 2011;14(2):141–6.
http://www.chemicalbook.com/ChemicalProductProperty_US_CB4260130.aspx.
https://www.lktlabs.com/product/atorvastatin-calcium-trihydrate/.
Lemsi M, Galai H, Louhaichi MR, Fessi H, Kalfat R. Amorphization of atorvastatin calcium by mechanical process: characterization and stabilization within polymeric matrix. J Pharm Innov. 2017;12:216–25. https://doi.org/10.1007/s12247-017-9282-0.
Dissertation—discovering new crystalline forms of atorvastatin calcium—new strategies for screening, Yong Suk Jin, https://d-nb.info/1029293406/34.
Ledeţi I, Ledeţi A, Vlase G, Vlase T, Matusz P, Bercean V, Suta L-M, Piciu D. Thermal stability of synthetic thyroid hormone l-thyroxine and l-thyroxine sodium salt hydrate both pure and in pharmaceutical formulations. J Pharm Biomed Anal. 2016;125:33–40.
Ilici M, Bercean V, Venter M, Ledeti I, Olariu T, Suta L-M, Fulias A. Investigations on the thermal-induced degradation of transitional coordination complexes containing (3 h-2-thioxo-1,3,4-thiadiazol-5-yl)thioacetate moiety. Rev Chim. 2014;65(10):1142–5.
Ledeţi I, Murariu M, Vlase G, Vlase T, Doca N, Ledeţi A, Şuta L-M, Olariu T. Investigation of thermal-induced decomposition of iodoform. J Therm Anal Calorim. 2017;127(1):565–70.
Suta LM, Vlase G, Ledeti A, Vlase T, Matusz P, Trandafirescu C, Circioban D, Olariu S, Ivan C, Murariu MS, Stelea L, Ledeti I. Solid-state thermal behaviour of cholic acid. Rev Chim. 2016;67(2):329–31.
Buda V, Andor M, Ledeti A, Ledeti I, Vlase G, Vlase T, Cristescu C, Voicu M, Suciu L, Tomescu M. Comparative solid-state stability of perindopril active substance vs. pharmaceutical formulation. Int J Mol Sci. 2017;18(1):164.
Ledeti A, Olariu T, Caunii A, Vlase G, Circioban D, Baul B, Ledeti I, Vlase T, Murariu M. Evaluation of thermal stability and kinetic of degradation for levodopa in non-isothermal conditions. J Therm Anal Calorim. 2017;1–8.
Ledeti I, Vlase G, Vlase T, Bercean V, Fulias A. Kinetic of solid-state degradation of transitional coordinative compounds containing functionalized 1,2,4-triazolic ligand. J Therm Anal Calorim. 2015;121(3):1049–57.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cristea, M., Baul, B., Ledeţi, I. et al. Preformulation studies for atorvastatin calcium. J Therm Anal Calorim 138, 2799–2806 (2019). https://doi.org/10.1007/s10973-019-08798-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08798-1