Abstract
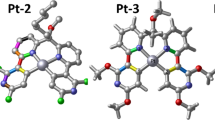
2-fluorobenzoic acid and 5,5′-dimethy-2,2′-bipyridine were used to construct two novel isostructural lanthanide complexes [Ln(2-FBA)3(5,5′-DM-2,2′-bipy)]2 (Ln = Eu(1), Tb(2); 2-FBA = 2-fluorobenzoate; 5,5′-DM-2,2′-bipy = 5,5′-dimethy-2,2′-bipyridine) by conventional solution method. They were characterized by infrared spectroscopy (IR), elemental analysis and single-crystal X-ray diffraction. The crystal description based on single-crystal X-ray diffraction data revealed that both of the complexes gave the triclinic crystal structure, belonging to the Pī space group. The two complexes were an infinite one-dimensional (1D) chain by hydrogen bonding (C–H…F) interactions to give a 2D supermolecular structure. Additionally, thermal behavior of the complexes was investigated by TG-DSC/FTIR technology in detail. The molar heat capacities of the two complexes in the temperature range of 278.15-423.15 K were determined by a DSC instrument, and their thermodynamic functions (HT-H298.15 K) and (ST-S298.15 K) were calculated. Beyond that, the fluorescence spectra and fluorescence lifetime of the complexes 1 and 2 were also evaluated.









Similar content being viewed by others
References
Kovalenko A, Rublev PO, Tcelykh LO, Goloveshkin AS, Lepnev LS, Burlov AS, Vashchenko AA, Marciniak Ł, Magerramov AM, Shikhaliyev NG, Vatsadze SZ, Utochnikova VV. Lanthanide complexes with 2-(Tosylamino)-benzylidene-N-(aryloyl)hydrazones: universal luminescent materials. Chem Mater. 2019;31(3):759–73. https://doi.org/10.1021/acs.chemmater.8b03675.
Ruiz C, García-Valdivia AA, Fernández B, Cepeda J, Oyarzabal I, Abas E, Laguna M, García JA, Fernández I, San Sebastian E, Rodríguez-Diéguez A. Multifunctional coordination compounds based on lanthanide ions and 5-bromonicotinic acid: magnetic, luminescence and anti-cancer properties. Cryst Eng Comm. 2019;21(25):3881–90. https://doi.org/10.1039/c9ce00292h.
Wu ZL, Lan XW, Zhang YX, Li M, Bai GY. Copper(i) iodide cluster-based lanthanide organic frameworks: synthesis and application as efficient catalysts for carboxylative cyclization of propargyl alcohols with CO2 under mild conditions. Dalton Trans. 2019;48(29):11063–9. https://doi.org/10.1039/c9dt01859j.
Lyubov DM, Tolpygin AO, Trifonov AA. Rare-earth metal complexes as catalysts for ring-opening polymerization of cyclic esters. Coord Chem Rev. 2019;392:83–145. https://doi.org/10.1016/j.ccr.2019.04.013.
Edelmann FT. Lanthanide amidinates and guanidinates: from laboratory curiosities to efficient homogeneous catalysts and precursors for rare-earth oxide thin films. Chem Soc Rev. 2009;38(8):2253–68. https://doi.org/10.1039/b800100f.
Eliseeva SV, Bünzli JCG. Lanthanide luminescence for functional materials and bio-sciences. Chem Soc Rev. 2010;39(1):189–227. https://doi.org/10.1039/b905604c.
Sabio RM, Santagneli SH, Gressier M, Caiut JMA, Pazin WM, Leite IS, Inada NM, Rosa da Silva R, Ribeiro SJL, Menu MJ. Luminescent nanohybrids based on silica and silylated Ru(II)-Yb(III) heterobinuclear complex: new tools for biological media analysis. Nat Nanotechnol. 2019;31(8):085709. https://doi.org/10.1088/1361-6528/ab55c3.
Bünzli JCG. Lanthanide light for biology and medical diagnosis. J Lumin. 2016;170:866–78. https://doi.org/10.1016/j.jlumin.2015.07.033.
Wu FS, Yang LL, Yue LX, Wang K, Liu GY, Luo XG, Zhu XJ. Ln(III) chelates-functionalized carbon quantum dots: synthesis, optical studies and multimodal bioimaging applications. Colloids Surf B Biointerfaces. 2019;175:272–80. https://doi.org/10.1016/j.colsurfb.2018.11.054.
Xu DZ, Liu MY, Huang Q, Chen JY, Huang HY, Deng FG, Wen YQ, Tian JW, Zhang XY, Wei Y. One-step synthesis of europium complexes containing polyamino acids through ring-opening polymerization and their potential for biological imaging applications. Talanta. 2018;188:1–6. https://doi.org/10.1016/j.talanta.2018.05.003.
Rossin A, Giambastiani G, Peruzzini M, Sessoli R. Amine-templated polymeric lanthanide formates: synthesis, characterization, and applications in luminescence and magnetism. Inorg Chem. 2012;51(12):6962–8. https://doi.org/10.1021/ic300854b.
Gao BJ, Qiao ZW, Chen T. Structure and photoluminescence property of complexes of aromatic carboxylic acid-functionalized polysulfone with Eu(III) and Tb(III). Mater Chem Phys. 2014;143(3):1119–30. https://doi.org/10.1016/j.matchemphys.2013.11.012.
Yan B. Recent progress in photofunctional lanthanide hybrid materials. RSC Adv. 2012;2(25):9304–24. https://doi.org/10.1039/c2ra20976d.
Chen FF, Chen ZQ, Bian ZQ, Huang CH. Sensitized luminescence from lanthanides in d–f bimetallic complexes. Coord Chem Rev. 2010;254(9–10):991–1010. https://doi.org/10.1016/j.ccr.2009.12.028.
Mara D, Artizzu F, Laforce B, Vincze L, Van Hecke K, Van Deun R, Kaczmarek AM. Novel tetrakis lanthanide β-diketonate complexes: structural study, luminescence properties and temperature sensing. J Lumin. 2019;213:343–55. https://doi.org/10.1016/j.jlumin.2019.05.035.
Xu LJ, Xu GT, Chen ZN. Recent advances in lanthanide luminescence with metal-organic chromophores as sensitizers. Coord Chem Rev. 2014;273–274:47–62. https://doi.org/10.1016/j.ccr.2013.11.021.
Gai YL, Jiang FL, Chen L, Wu MY, Su KZ, Pan J, Wan XY, Hong MC. Europium and terbium coordination polymers assembled from hexacarboxylate ligands: structures and luminescent properties. Cryst Growth Des. 2014;14(3):1010–7. https://doi.org/10.1021/cg401452p.
Feng R, Jiang FL, Wu MY, Chen L, Yan CF, Hong MC. Structures and photoluminescent properties of the lanthanide coordination complexes with hydroxyquinoline carboxylate ligands. Cryst Growth Des. 2010;10(5):2306–13. https://doi.org/10.1021/cg100026d.
ManjuBala Kumar S, Chahar S, Taxak VB, Boora P, Khatkar SP. Synthesis, NMR and optical features of intense green color terbium(III) complexes. Optik. 2020;202:163636. https://doi.org/10.1016/j.ijleo.2019.163636.
Ren N, Wang F, Zhang JJ, Zheng XF. Progress in thermal analysis kinetics. Acta Phys-Chim Sin. 2020;36(6):1905062. https://doi.org/10.3866/PKU.WHXB201905062.
Wang JJ, Zhang JJ. Thermal analysis kinetics and thermokinetics. Acta Phys-Chim Sin. 2020;36(6):1909020. https://doi.org/10.3866/PKU.WHXB201909020.
Qiao CF, Lü L, Xu WF, Xia ZQ, Zhou CS, Chen SP, Gao SL. Synthesis, thermal decomposition kinetics and detonation performance of a three-dimensional solvent-free energetic Ag(I)-MOF. Acta Phys-Chim Sin. 2020;36(6):1905085. https://doi.org/10.3866/PKU.WHXB201905085.
Wu XH, Ren N, Zhang JJ, Wang DQ. Lanthanide complexes with 2-bromo-5-methoxybenzoic acid and 5,5'-dimethyl-2,2'-bipyridine: crystal structures, thermodynamic properties and luminescence behaviors. J Chem Thermodyn. 2018;123:99–106. https://doi.org/10.1016/j.jct.2018.04.002.
Sologubov SS, Markin AV, Sarmini YA, Samosudova YS, Smirnova NN, Boldyrev KL, Tatarinova EA, Meshkov IB, Muzafarov AM. Calorimetric study of siloxane dendrimer of the third generation with trimethylsilyl terminal groups. J Therm Anal Calorim. 2019;138(5):3301–10. https://doi.org/10.1007/s10973-019-08693-9.
Pooley LI, Abu-Bakar AS, Cran MJ, Wadhwani R, Moinuddin KAM. Measurements of specific heat capacity of common building materials at elevated temperatures: a comparison of DSC and HAD. J Therm Anal Calorim. 2019;141(4):1279–89. https://doi.org/10.1007/s10973-019-09124-5.
Fang DW, Gong L, Fan XT, Liang KH, Ma XX, Wei J. Low-temperature heat capacity and standard thermodynamic functions of the novel ionic liquid 1-(2-methoxyethyl)-3-ethyl imidazolium perrhenate. J Therm Anal Calorim. 2019;138(2):1437–42. https://doi.org/10.1007/s10973-019-08295-5.
Marques LF, Cantaruti AAB, Correa CC, Lahoud MG, da Silva RR, Ribeiro SJL, Machado FC. First crystal structures of lanthanide-hydrocinnamate complexes: hydrothermal synthesis and photophysical studies. J Photochem Photobiol A. 2013;252:69–76. https://doi.org/10.1016/j.jphotochem.2012.11.012.
Wang CY, Kang J, Zhang XQ, Zhao YL, Chu HB. Crystal structures and luminescence properties of lanthanide complexes with 4-bromobenzoate and nitrogen heterocyclic ligands. J Lumin. 2019;215:116638. https://doi.org/10.1016/j.jlumin.2019.116638.
Taha ZA, Ajlouni AM, Al Momani W, Al-Ghzawi AA. Syntheses, characterization, biological activities and photophysical properties of lanthanides complexes with a tetradentate schiff base ligand. Spectrochim Acta A Mol Biomol Spectrosc. 2011;81(1):570–7. https://doi.org/10.1016/j.saa.2011.06.052.
Shi J, Hou YJ, Chu WY, Shi XH, Gu HQ, Wang BL, Sun ZZ. Crystal structure and highly luminescent properties studies of bis-beta-diketonate lanthanide complexes. Inorg Chem. 2013;52(9):5013–22. https://doi.org/10.1021/ic302726z.
Zhu MM, Ren N, Zhang JJ, Wang DQ. Construction of three types of lanthanide complexes based on 3,4-dimethylbenzoic acid and 5,5′-dimethyl-2,2′-bipyridine: syntheses, structures, thermodynamic properties, luminescence, and bacteriostatic activities. Appl Organomet Chem. 2018;32(9):e4438. https://doi.org/10.1002/aoc.4438.
Li YY, Ren N, He SM, Zhang JJ. Supramolecular structures, thermal decomposition mechanism and heat capacity of the novel binuclear Tb(III) and Dy(III) complexes with 2,3-dimethoxybenzoic acid and 5,5′-dimety-2,2′-bipyridine. J Therm Anal Calorim. 2019;140(5):2435–45. https://doi.org/10.1007/s10973-019-08944-9.
Liu QS, Tan ZC, Welz-Biermann U, Liu XX. Molar heat capacity and thermodynamic properties of N-alklypyridinium hexafluorophosphate salts, [Cnpy][PF6] (n=2, 3, 5). J Chem Thermodyn. 2014;68:82–9. https://doi.org/10.1016/j.jct.2013.08.024.
He DH, Di YY, Wang B, Dan WY, Tan ZC. Low-temperature heat capacities and thermodynamic properties of ethylenediammonium tetrachlorozincate chloride (C2H10N2)2(ZnCl4)Cl2. Thermochim Acta. 2010;506(1–2):41–6. https://doi.org/10.1016/j.tca.2010.04.012.
Kotyk CM, Weber JE, Hyre AS, McNeely J, Monteiro J, Domin M, Balaich GJ, Rheingold AL, de Bettencourt-Dias A, Doerrer LH. Luminescence of lanthanide complexes with perfluorinated alkoxide ligands. Inorg Chem. 2020;59(14):9807–23. https://doi.org/10.1021/acs.inorgchem.0c00782.
Zhou MX, Ren N, Zhang JJ. Crystal structure, thermal decomposition mechanism and properties of lanthanide supramolecular complexes based on 2,4,6-trimethylbenzoic acid and 5,5’-dimethyl-2,2’-bipyridine. Acta Phys -Chim Sin. 2021;37:2004071. https://doi.org/10.3866/PKU.WHXB202004071.
Wang XQ, Zhang LL, Yang J, Liu FL, Dai FN, Wang RM, Sun DF. Lanthanide metal–organic frameworks containing a novel flexible ligand for luminescence sensing of small organic molecules and selective adsorption. J Mater Chem A. 2015;3(24):12777–85. https://doi.org/10.1039/c5ta00061k.
Kukinov AA, Balashova TV, Ilichev VA, Trufanov AN, Ivin MN, Obolensky SV, Bochkarev MN. X-Ray excited luminescence of organo-lanthanide complexes. Phys Chem Chem Phys. 2019;21(29):16288–92. https://doi.org/10.1039/c9cp03041g.
Sangeetha P, Jayaprakash P, Ramesh P, Sudha S, Vinitha G, Nageshwari M, Caroline ML. Crystal growth, spectroscopic, optical, thermal and hirshfeld surface analysis of glycinium hydrogen fumarate glycine solvate monohydrate (GHFGSM): a third harmonic nonlinear optical organic crystal. J Mol Struct. 2020;1213:128187. https://doi.org/10.1016/j.molstruc.2020.128187.
Li HL, Liu YJ, Zheng R, Ma X, Chen LJ, Zhao JW. Syntheses, structures and fluorescence properties of three rare-earth containing docosatungstates. Spectrochim Acta A Mol Biomol Spectrosc. 2017;176:114–22. https://doi.org/10.1016/j.saa.2017.01.016.
Acknowledgements
The research work was supported by the National Natural Science Foundation of China (No. 21803016).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Du, DD., Ren, N. & Zhang, JJ. Syntheses, crystal structures, thermodynamic and fluorescent properties of dinuclear lanthanide complexes constructed with 2-fluorobenzoic acid and 5,5′-dimethy-2,2′-bipyridine. J Therm Anal Calorim 147, 1–10 (2022). https://doi.org/10.1007/s10973-021-10563-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-021-10563-2