Abstract
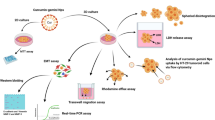
The therapeutic application of recombinant proteins is limited due to their inherent structural complexity. Additionally, screening of therapeutic potential of protein products requires an appropriate testing platform to achieve biological relevance. Fabrication of three dimensional cultures bridges the gap between in vitro based monolayer cultures and clinical applications. In this perspective, glioblastoma U-87 MG and breast cancer MCF7 spheroids were generated to assess the therapeutic prospect of recombinant PTEN protein. PTEN bound to silver nanoclusters was encapsulated within PEG coating, which resulted in fabrication of spherical nanocarriers named as PTEN-nanocomposites. Internalization of PTEN-nanocomposites in the spheroids was confirmed by confocal microscopy. Upon uptake, PTEN-nanocomposites led to modulation of cyclins and apoptosis gene regulators culminating in cell cycle arrest and reduced cell viability as confirmed by calcein-AM/PI dual staining and alamar blue assay. Further, combination of tamoxifen and PTEN-nanocomposites on U-87 MG spheroids resulted in two-fold reduction of drug dosage. The study revealed that the monolayer culture results translated to the 3D culture as well, however higher dose of the recombinant PTEN was required for the spheroid system. The anti-proliferative role of PTEN-nanocomposites in a complex 3D environment augments its biological implication and paves the way for recombinant PTEN based therapeutic applications.






Similar content being viewed by others
References
Fennema E, Rivron N, Rouwkema J, van Blitterswijk C, de Boer J (2013) Spheroid culture as a tool for creating 3D complex tissues. Trends Biotechnol 31:108–115
Pampaloni F, Reynaud EG, Stelzer EHK (2007) The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol 8:839
Cunha C, Panseri S, Villa O, Silva D, Gelain F (2011) 3D culture of adult mouse neural stem cells within functionalized self-assembling peptide scaffolds. Int J Nanomed 6:943–955
Owen SC, Shoichet MS (2010) Design of three-dimensional biomimetic scaffolds. J Biomed Mater Res, Part A 94:1321–1331
Breslin S, O’Driscoll L (2013) Three-dimensional cell culture: the missing link in drug discovery. Drug Discov Today 18:240–249
Amaral RLF, Miranda M, Marcato PD, Swiech K (2017) Comparative analysis of 3D bladder tumor spheroids obtained by forced floating and hanging drop methods for drug screening. Front Physiol 8:605
Frey O, Misun PM, Fluri DA, Hengstler JG, Hierlemann A (2014) Reconfigurable microfluidic hanging drop network for multi-tissue interaction and analysis. Nat Commun 5:4250
Zanoni M, Piccinini F, Arienti C, Zamagni A, Santi S, Polico R et al (2016) 3D tumor spheroid models for in vitro therapeutic screening: a systematic approach to enhance the biological relevance of data obtained. Sci Rep 6:19103
Riedl A, Schlederer M, Pudelko K, Stadler M, Walter S, Unterleuthner D et al (2017) Comparison of cancer cells in 2D vs 3D culture reveals differences in AKT–mTOR–S6 K signaling and drug responses. J Cell Sci 130:203
Song MS, Salmena L, Pandolfi PP (2012) The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol 13:283–296
Hollander MC, Blumenthal GM, Dennis PA (2011) PTEN loss in the continuum of common cancers, rare syndromes and mouse models. Nat Rev Cancer 11:289–301
Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T et al (1998) Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell 95:29–39
Papa A, Chen M, Pandolfi PP (2013) Pills of PTEN? In and out for tumor suppression. Cell Res 23:1155–1156
Arora N, Gavya SL, Ghosh SS (2018) Multi-facet implications of PEGylated lysozyme stabilized-silver nanoclusters loaded recombinant PTEN cargo in cancer theranostics. Biotechnol Bioeng 115:1116–1127
Arora N, Ghosh SS (2016) Functional characterizations of interactive recombinant PTEN-silica nanoparticles for potential biomedical applications. RSC Adv 6:114944–114954
Zhou T, Huang Y, Li W, Cai Z, Luo F, Yang CJ et al (2012) Facile synthesis of red-emitting lysozyme-stabilized Ag nanoclusters. Nanoscale 4:5312–5315
Ivascu A, Kubbies M (2006) Rapid generation of single-tumor spheroids for high-throughput cell function and toxicity analysis. J Biomol Screen 11:922–932
Friedrich J, Seidel C, Ebner R, Kunz-Schughart LA (2009) Spheroid-based drug screen: considerations and practical approach. Nat Protoc 4:309–324
Walzl A, Unger C, Kramer N, Unterleuthner D, Scherzer M, Hengstschlager M et al (2014) The resazurin reduction assay can distinguish cytotoxic from cytostatic compounds in spheroid screening assays. J Biomol Screen 19:1047–1059
Riccardi C, Nicoletti I (2006) Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat Protoc 1:1458–1461
Froehlich K, Haeger JD, Heger J, Pastuschek J, Photini SM, Yan Y et al (2016) Generation of multicellular breast cancer tumor spheroids: comparison of different protocols. J Mammary Gland Biol Neoplasia 21:89–98
Collazo A, Bricaud O, Desai K (2005) Use of confocal microscopy in comparative studies of vertebrate morphology. Methods Enzymol 395:521–543
Chan LL, Wilkinson AR, Paradis BD, Lai N (2012) Rapid image-based cytometry for comparison of fluorescent viability staining methods. J Fluoresc 22:1301–1311
Maddika S, Ande SR, Wiechec E, Hansen LL, Wesselborg S, Los M (2008) Akt-mediated phosphorylation of CDK2 regulates its dual role in cell cycle progression and apoptosis. J Cell Sci 121:979–988
Hiraoka D, Aono R, Hanada S-I, Okumura E, Kishimoto T (2016) Two new competing pathways establish the threshold for cyclin-B-Cdk1 activation at the meiotic G2/M transition. J Cell Sci 129:3153–3166
Katsuno Y, Suzuki A, Sugimura K, Okumura K, Zineldeen DH, Shimada M et al (2009) Cyclin A-Cdk1 regulates the origin firing program in mammalian cells. Proc Natl Acad Sci 106:3184
Bendris N, Lemmers B, Blanchard J-M, Arsic N (2011) Cyclin A2 mutagenesis analysis: a new insight into CDK activation and cellular localization requirements. PLoS ONE 6:e22879
Westphal D, Dewson G, Czabotar PE, Kluck RM (2011) Molecular biology of Bax and Bak activation and action. Biochem Biophys Acta 1813:521–531
Tsuruta F, Masuyama N, Gotoh Y (2002) The phosphatidylinositol 3-kinase (PI3 K)-Akt Pathway suppresses Bax translocation to mitochondria. J Biol Chem 277:14040–14047
Dansen TB, Burgering BMT (2008) Unravelling the tumor-suppressive functions of FOXO proteins. Trends Cell Biol 18:421–429
Fu Z, Tindall DJ (2008) FOXOs, cancer and regulation of apoptosis. Oncogene 27:2312
Zhang X, Tang N, Hadden TJ, Rishi AK (2011) Akt, FoxO and regulation of apoptosis. Biochem Biophys Acta 1813:1978–1986
Medema RH, Kops GJPL, Bos JL, Burgering BMT (2000) AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature 404:782
Huang Y, Sramkoski RM, Jacobberger JW (2013) The kinetics of G2 and M transitions regulated by B cyclins. PLoS ONE 8:e80861
Krishan A (1975) Rapid flow cytofluorometric analysis of mammalian cell cycle by propidium iodide staining. J Cell Biol 66:188
Acknowledgements
Financial supports of the Department of Biotechnology, Government of India, for the NER-BPMC (BT/PR 25095/NER/95/1011/2017), DBT Program Support (BT/PR13560/COE/34/44/2015), partial support of the Department of Electronics and Information Technology (No. 5(9)/2012-NANO (Vol. II)), Government of India are acknowledged. Authors also acknowledge the support of the Central Instruments Facility (CIF) and the Centre for Nanotechnology, IIT Guwahati for providing instrumentation facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest with others regarding this current work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arora, N., Shome, R. & Ghosh, S.S. Deciphering therapeutic potential of PEGylated recombinant PTEN-silver nanoclusters ensemble on 3D spheroids. Mol Biol Rep 46, 5103–5112 (2019). https://doi.org/10.1007/s11033-019-04965-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-019-04965-7