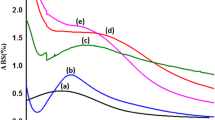
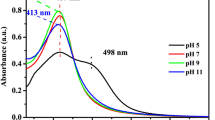
Abstract
The size and shape of silver nanoparticles (AgNPs) can potentially influence their antibacterial activity. In this study, a photochemical approach was adopted for the synthesis of decahedral AgNPs and their antibacterial activity was tested and compared against that of spherical AgNPs of similar size synthesized using the chemical reduction approach. The UV–vis spectra indicated the synthesis of decahedral AgNPs with a localized surface plasmon resonance (LSPR) peak at 502 nm. The spherical AgNPs exhibited the LSPR peak at 416 nm. Analysis of field emission gun-transmission electron microscopy (FEG-TEM) micrographs demonstrated the average diameter of decahedral silver nanoparticles as 52.1 ± 5.7 nm with side length as 33.2 ± 3.1 nm. In contrast, the average size of spherical AgNPs was 44.2 ± 6.3 nm. The decahedral AgNPs demonstrated ten times higher bactericidal activity as compared to the spherical AgNPs across all the bacterial strains tested. The minimum inhibitory concentration (MIC) for decahedral AgNPs was in the range of 4–8 μg/ml for all the four bacterial strains tested, whereas, for spherical nanoparticles of comparable size, the MIC was in the range of 40–80 μg/ml. The minimum bactericidal concentration (MBC) for decahedral AgNPs was in the range of 6–10 μg/ml. At the same time, spherical AgNPs of comparable size exhibited MBC in the range of 60–100 μg/ml for the four bacterial strains. In terms of bactericidal effect, Escherichia coli MTCC 443 was found as the most sensitive strain, while in terms of growth inhibition, Bacillus subtilis was the most sensitive strain. Staphylococcus aureus NCIM 5021 was the most resistant among the tested bacterial strains.







Similar content being viewed by others
Data availability
Not applicable.
References
Agnihotri S, Mukherji S, Mukherji S (2013) Immobilized silver nanoparticles enhance contact killing and show highest efficacy: elucidation of the mechanism of bactericidal action of silver. Nanoscale 5:7328–7340. https://doi.org/10.1039/C3NR00024A
Agnihotri S, Mukherji S, Mukherji S (2014) Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. RSC Adv 4:3974–3983. https://doi.org/10.1039/c3ra44507k
Araújo P, Lemos M, Mergulhão F, et al (2011) Antimicrobial resistance to disinfectants in biofilms. In: Science against microbial pathogens: communicating current research and technological advances, A. Méndez-. pp 826–834
Ashbolt NJ (2015) Microbial contamination of drinking water and human health from community water systems. Curr Environ Heal reports 2:95–106
Bae E, Park HJ, Lee J, Kim Y, Yoon J, Park K, Choi K, Yi J (2010) Bacterial cytotoxicity of the silver nanoparticle related to physicochemical metrics and agglomeration properties. Environ Toxicol Chem 29:2154–2160. https://doi.org/10.1002/etc.278
Bharti S, Agnihotri S, Mukherji S, Mukherji S (2015) Effectiveness of immobilized silver nanoparticles in inactivation of pathogenic bacteria. J Environ Res Dev 9:849–856
Casals E, Pfaller T, Duschl A, Oostingh GJ, Puntes V (2010) Time evolution of the nanoparticle protein corona. ACS Nano 4:3623–3632
Chakraborty S, Mukherji S, Mukherji S (2010) Surface hydrophobicity of petroleum hydrocarbon degrading Burkholderia strains and their interactions with NAPLs and surfaces. Colloids Surf B: Biointerfaces 78:101–108
Chang S, Chen K, Hua Q, Ma Y, Huang W (2011) Evidence for the growth mechanisms of silver nanocubes and nanowires. J Phys Chem C 115:7979–7986. https://doi.org/10.1021/jp2010088
Cheon JY, Kim SJ, Rhee YH, Kwon OH, Park WH (2019) Shape-dependent antimicrobial activities of silver nanoparticles. Int J Nanomedicine 14:2773–2780. https://doi.org/10.2147/IJN.S196472
Chudasama B, Vala AK, Andhariya N, Upadhyay RV, Mehta RV (2009) Enhanced antibacterial activity of bifunctional Fe3O4–Ag core – shell nanostructures. Nano Res 2:955–965. https://doi.org/10.1007/s12274-009-9098-4
Dasgupta N, Ranjan S, Rajendran B et al (2015) Thermal co-reduction approach to vary size of silver nanoparticle: its microbial and cellular toxicology. Environ Sci Pollut Res 23:4149–4163
Dasgupta D, Jasmine J, Mukherji S (2018) Characterization, phylogenetic distribution and evolutionary trajectories of diverse hydrocarbon degrading microorganisms isolated from refinery sludge. 3 biotech 8:1–18. https://doi.org/10.1007/s13205-018-1297-9
El Badawy AM, Silva RG, Morris B et al (2011) Surface charge-dependent toxicity of silver nanoparticles. Environ Sci Technol 45:283–287. https://doi.org/10.1021/es1034188
Franci G, Falanga A, Galdiero S, Palomba L, Rai M, Morelli G, Galdiero M (2015) Silver nanoparticles as potential antibacterial agents. Molecules 20:8856–8874. https://doi.org/10.3390/molecules20058856
Goyal D, Kaur G, Tewari R, Kumar R (2017) Correlation of edge truncation with antibacterial activity of plate-like anisotropic silver nanoparticles. Environ Sci Pollut Res 24:20429–20437. https://doi.org/10.1007/s11356-017-9630-0
Guzmán MG, Dille J, Godet S (2009) Synthesis of silver nanoparticles by chemical reduction method and their antibacterial activity. Int J Chem Biomol Eng 2:104–111
Helmlinger J, Sengstock C, Groß-Heitfeld C, Mayer C, Schildhauer TA, Köller M, Epple M (2016) Silver nanoparticles with different size and shape: equal cytotoxicity, but different antibacterial effects. RSC Adv 6:18490–18501. https://doi.org/10.1039/C5RA27836H
Hong X, Wen J, Xiong X, Hu Y (2016) Shape effect on the antibacterial activity of silver nanoparticles synthesized via a microwave-assisted method. Environ Sci Pollut Res 23:4489–4497. https://doi.org/10.1007/s11356-015-5668-z
Iravani S, Korbekandi H, Mirmohammadi SV, Zolfaghari B (2014) Synthesis of silver nanoparticles: chemical , physical and biological methods. Res Pharm Sci 9:385–406
Ito A, Taniuchi A, May T, Kawata K, Okabe S (2009) Increased antibiotic resistance of Escherichia coli in mature biofilms. Appl Environ Microbiol 75:4093–4100. https://doi.org/10.1128/AEM.02949-08
Jose M, Thanal R, Prince Joshua J, Martin Britto Dhas SA, Jerome Das S (2016) Anisotropic growth of silver nanostructures from silver spheres by a simple chemical reduction route. Superlattice Microst 89:68–74. https://doi.org/10.1016/j.spmi.2015.10.039
Keren I, Kaldalu N, Spoering A, Wang Y, Lewis K (2004) Persister cells and tolerance to antimicrobials. FEMS Microbiol Lett 230:13–18. https://doi.org/10.1016/S0378-1097(03)00856-5
Keunen R, Cathcart N, Kitaev V (2014) Plasmon mediated shape and size selective synthesis of icosahedral silver nanoparticles via oxidative etching and their 1-D transformation to pentagonal pins. Nanoscale 6:8045–8051
Lee GP, Shi Y, Lavoie E, Daeneke T, Reineck P, Cappel UB, Huang DM, Bach U (2013) Light-driven transformation processes of anisotropic silver nanoparticles. ACS Nano 7:5911–5921. https://doi.org/10.1021/nn4013059
Li J, Rong K, Zhao H, Li F, Lu Z, Chen R (2013) Highly selective antibacterial activities of silver nanoparticles against Bacillus subtilis. J Nanosci Nanotechnol 13:6806–6813. https://doi.org/10.1166/jnn.2013.7781
Li K, Wu Q, Xu T, Kang Q, Yao M, Song G, Lin Y, Chen Z, Zheng T (2016) Silver nanoparticles with different morphologies: growth mechanism and stability. Mater Res Innov 20:58–66. https://doi.org/10.1080/14328917.2015.1130381
Li J, Xie S, Ahmed S, Wang F, Gu Y, Zhang C, Chai X, Wu Y, Cai J, Cheng G (2017) Antimicrobial activity and resistance: influencing factors. Front Pharmacol 8:1–11. https://doi.org/10.3389/fphar.2017.00364
Maillard M, Huang P, Brus L (2003) Silver nanodisk growth by surface plasmon enhanced photoreduction of adsorbed [Ag+]. Nano Lett 3:1611–1615. https://doi.org/10.1021/nl034666d
Mlalila NG, Swai HS, Hilonga A, Kadam DM (2016) Antimicrobial dependence of silver nanoparticles on surface plasmon resonance bands against Escherichia coli. Nanotechnol Sci Appl 10:1–9. https://doi.org/10.2147/NSA.S123681
Mohanty S, Mukherji S (2012) Alteration in cell surface properties of Burkholderia spp. during surfactant aided biodegradation of petroleum hydrocarbons. Appl Microbiol Biotechnol 94:193–204
Mukherji S, Ruparelia J, Agnihotri S (2012) Antimicrobial activity of silver and copper nanoparticles: variation in sensitivity across various strains of bacteria and fungi. In: Nano-antimicrobials: progesses and prospects. Springer, Berlin Heidelberg, pp 225–251
Mukherji S, Bharti S, Shukla G, Mukherji S (2018) Synthesis and characterization of size- and shape-controlled silver nanoparticles. Phys Sci Rev 4
Murph SEH, Larsen GK, Coopersmith KJ (2017) Anisotropic and shape-selective nanomaterials: structure-property relationships. Springer International Publishing AG
Murshid N (2013) Controlled transformation of nanoparticles with tunable surface plasmon resonance controlled transformation of nanoparticles with tunable surface plasmon resonance by submitted to the Department of Chemistry. Wilfrid Laurier University
Navaladian S, Viswanathan B, Varadarajan TK, Viswanath RP (2008) Microwave-assisted rapid synthesis of anisotropic Ag nanoparticles by solid state transformation. Nanotechnology 19. https://doi.org/10.1088/0957-4484/19/04/045603
Pal S, Tak YK, Song JM (2007) Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the Gram-negative bacterium Escherichia coli. Appl Environ Microbiol 73:1712–1720. https://doi.org/10.1128/AEM.02218-06
Pandey PK, Kass PH, Soupir ML et al (2014) Contamination of water resources by pathogenic bacteria. AMB Express 4:1–16
Pencheva D, Bryaskova R, Kantardjiev T (2012) Polyvinyl alcohol/silver nanoparticles (PVA/AgNps) as a model for testing the biological activity of hybrid materials with included silver nanoparticles. Mater Sci Eng C 32:2048–2051
Pietrobon B, Kitaev V (2008) Photochemical synthesis of monodisperse size-controlled silver decahedral nanoparticles and their remarkable optical properties. Chem Mater 20:5186–5190
Pietrobon B, McEachran M, Kitaev V (2009) Synthesis of size-controlled faceted pentagonal silver nanorods with tunable plasmonic properties and self-assembly of these nanorods. ACS Nano 3:21–26. https://doi.org/10.1021/nn800591y
Qi Y, Zhang T, Jing C, Liu S, Zhang C, Alvarez PJJ, Chen W (2020) Nanocrystal facet modulation to enhance transferrin binding and cellular delivery. Nat Commun 11:1–10. https://doi.org/10.1038/s41467-020-14972-z
Rai M, Yadav A, Gade A (2009) Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv 27:76–83
Raza MA, Kanwal Z, Rauf A, Sabri A, Riaz S, Naseem S (2016) Size- and shape-dependent antibacterial studies of silver nanoparticles synthesized by wet chemical routes. Nanomaterials 6:1–15. https://doi.org/10.3390/nano6040074
Roh J, Umh HN, Sim J, Park S, Yi J, Kim Y (2013) Dispersion stability of citrate- and PVP-coated AgNPs in biological media for cytotoxicity test. Korean J Chem Eng 30:671–674. https://doi.org/10.1007/s11814-012-0172-3
Rojas-Andrade M, Cho AT, Hu P, Lee SJ, Deming CP, Sweeney SW, Saltikov C, Chen S (2015) Enhanced antimicrobial activity with faceted silver nanostructures. J Mater Sci 50:2849–2858
Ruparelia JP, Chatterjee AK, Duttagupta SP, Mukherji S (2008) Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater 4:707–716
Saade J, De Araújo CB (2014) Synthesis of silver nanoprisms: a photochemical approach using light emission diodes. Mater Chem Phys 148:1184–1193. https://doi.org/10.1016/j.matchemphys.2014.09.045
Shannahan JH, Lai X, Ke PC, Podila R, Brown JM, Witzmann FA (2013) Silver nanoparticle protein corona composition in cell culture media. PLoS One 8:e74001. https://doi.org/10.1371/journal.pone.0074001
Silvestry-rodriguez N, Sicairos-ruelas EE, Gerba CP, Bright KR (2007) Silver as a disinfectant. Rev Environ Contam Toxicol 191:23–45. https://doi.org/10.1007/978-0-387-69163-3
Sondi I, Salopek-Sondi B (2004) Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci 275:177–182
Stamplecoskie K, Scaiano J (2010) Light emitting diode irradiation can control the morphology and optical properties of silver nanoparticles. J Am Chem Soc 8:1825–1827. https://doi.org/10.1021/ja910010b
Valodkar M, Modi S, Pal A, Thakore S (2011) Synthesis and anti-bacterial activity of Cu, Ag and Cu-Ag alloy nanoparticles: a green approach. Mater Res Bull 46:384–389
Vellora V, Padil T, Černík M (2013) Green synthesis of copper oxide nanoparticles using gum karaya as a biotemplate and their antibacterial application. Int J Nanomedicine:889–898
Xie ZX, Tzeng WC, Huang CL (2016) One-pot synthesis of icosahedral silver nanoparticles by using a photoassisted tartrate reduction method under UV light with a wavelength of 310 nm. ChemPhysChem:2551–2557. https://doi.org/10.1002/cphc.201600257
Yang X, Fu H, Jiang X, Yu A (2013) Silver nanoparticles: synthesis, growth, mechanism and bioapplications. In: Silver nanoparticles: synthesis, Uses and Health Concerns. Nova Science Publishers, Inc., pp 395–460
Yoon KY, Hoon Byeon J, Park JH, Hwang J (2007) Susceptibility constants of Escherichia coli and Bacillus subtilis to silver and copper nanoparticles. Sci Total Environ 373:572–575. https://doi.org/10.1016/j.scitotenv.2006.11.007
Zhang R, Kang Y, Xie B (2015) Assembly and antibacterial activity of horizontally oriented silver nanoplates. J Appl Polym Sci 132. https://doi.org/10.1002/app.42070
Zheng X, Zhao X, Guo D, Tang B, Xu S, Zhao B, Xu W, Lombardi JR (2009) Photochemical formation of silver nanodecahedra: structural selection by the excitation wavelength. Langmuir 25:3802–3807. https://doi.org/10.1021/la803814j
Acknowledgments
The authors would like to acknowledge the Sophisticated Analytical Instrument Facility (SAIF), IIT Bombay, for providing FEG-TEM, FEG-SEM, and ICP-AES facilities and the Department of Metallurgy Engineering and Materials Science (MEMS), IIT Bombay, for providing the DLS measurement and zeta potential analyzer for characterization of the nanoparticles. The authors would also like to thank Dr. Gauri Shukla for assisting in the preparation of the set-up for photochemical synthesis of decahedral silver nanoparticles.
Author information
Authors and Affiliations
Contributions
Sharda Bharti performed all the experiments, analyzed the results, and prepared the manuscript. Prof. Suparna Mukherji supervised the work and revised the manuscript. Prof. Soumyo Mukherji co-supervised the research work. All authors discussed the experiments and the results and contributed to the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 411 kb).
Rights and permissions
About this article
Cite this article
Bharti, S., Mukherji, S. & Mukherji, S. Enhanced antibacterial activity of decahedral silver nanoparticles. J Nanopart Res 23, 36 (2021). https://doi.org/10.1007/s11051-020-05106-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-020-05106-z