Abstract
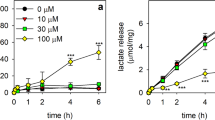
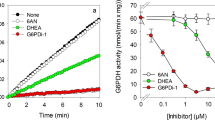
The reduction of water-soluble tetrazolium salts (WSTs) is frequently used to determine the metabolic integrity and the viability of cultured cells. Recently, we have reported that the electron cycler menadione can efficiently connect intracellular oxidation reactions in cultured astrocytes with the extracellular reduction of WST1 and that this menadione cycling reaction involves an enzyme. The enzymatic reaction involved in the menadione-dependent WST1 reduction was found strongly enriched in the cytosolic fraction of cultured astrocytes and is able to efficiently use both NADH and NADPH as electron donors. In addition, the reaction was highly sensitive towards dicoumarol with Kic values in the low nanomolar range, suggesting that the NAD(P)H:quinone oxidoreductase 1 (NQO1) catalyzes the menadione-dependent WST1 reduction in astrocytes. Also, in intact astrocytes, dicoumarol inhibited the menadione-dependent WST1 reduction in a concentration-dependent manner with half-maximal inhibition observed at around 50 nM. Moreover, the menadione-dependent WST1 reduction by viable astrocytes was strongly affected by the availability of glucose. In the absence of glucose only residual WST1 reduction was observed, while a concentration-dependent increase in WST1 reduction was found during a 30 min incubation with maximal WST1 reduction already determined in the presence of 0.5 mM glucose. Mannose could fully replace glucose as substrate for astrocytic WST1 reduction, while other hexoses, lactate and the mitochondrial substrate β-hydroxybutyrate failed to provide electrons for the cell-dependent WST1 reduction. These results demonstrate that the menadione-mediated WST1 reduction involves cytosolic NQO1 activity and that this process is strongly affected by the availability of glucose as metabolic substrate.





Similar content being viewed by others
References
Berridge MV, Herst PM, Tan AS (2005) Tetrazolium dyes as tools in cell biology: new insights into their cellular reduction. Biotechnol Annu Rev 11:127–152
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
van Tonder A, Joubert AM, Cromarty AD (2015) Limitations of the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay when compared to three commonly used cell enumeration assays. BMC Res Notes 8:47
Ishiyama M, Shiga M, Sasamoto K, Mizoguchi M, He P-G (1993) A new sulfonated tetrazolium salt that produces a highly water-soluble formazan dye. Chem Pharm Bull 41:1118–1122
Ishiyama M, Tominaga H, Shiga M, Sasamoto K, Ohkura Y, Ueno K (1996) A combined assay of cell vability and in vitro cytotoxicity with a highly water-soluble tetrazolium salt, neutral red and crystal violet. Biol Pharm Bull 19:1518–1520
Tan AS, Berridge MV (2004) Distinct trans-plasma membrane redox pathways reduce cell-impermeable dyes in HeLa cells. Redox Rep 9:302–306
Gerard E, Spengler RN, Bonoiu AC, Mahajan SD, Davidson BA, Ding H, Kumar R, Prasad PN, Knight PR, Ignatowski TA (2015) Chronic constriction injury-induced nociception is relieved by nanomedicine-mediated decrease of rat hippocampal tumor necrosis factor. Pain 156:1320–1333
Stapelfeldt K, Ehrke E, Steinmeier J, Rastedt W, Dringen R (2017) Menadione-mediated WST1 reduction assay for the determination of metabolic activity of cultured neural cells. Anal Biochem 538:42–52
Dinkova-Kostova AT, Talalay P (2010) NAD(P)H:quinone acceptor oxidoreductase 1 (NQO1), a multifunctional antioxidant enzyme and exceptionally versatile cytoprotector. Arch Biochem Biophys 501:116–123
Tsukatani T, Suenaga H, Higuchi T, Akao T, Ishiyama M, Ezoe K, Matsumoto K (2008) Colorimetric cell proliferation assay for microorganisms in microtiter plate using water-soluble tetrazolium salts. J Microbiol Methods 75:109–116
Argente-Arizón P, Guerra-Cantera S, Garcia-Segura LM, Argente J, Chowen JA (2017) Glial cells and energy balance. J Mol Endocrinol 58:59–71
Jäkel S, Dimou L (2017) Glial cells and their function in the adult brain: a journey through the history of their ablation. Front Cell Neurosci 11:24
Bolaños JP (2016) Bioenergetics and redox adaptations of astrocytes to neuronal activity. J Neurochem 139(Suppl 2):115–125
Schmidt MM, Dringen R (2012) Glutathione (GSH) synthesis and metabolism. In: Choi I-Y, Gruetter R (eds) Neural metabolism in vivo. Springer, Boston, pp 1029–1050
Dringen R, Brandmann M, Hohnholt MC, Blumrich E-M (2015) Glutathione-dependent detoxification processes in astrocytes. Neurochem Res 40:2570–2582
Magistretti PJ, Allaman I (2018) Lactate in the brain: from metabolic end-product to signalling molecule. Nat Rev Neurosci 19:235
Bouzier-Sore AK, Pellerin L (2013) Unraveling the complex metabolic nature of astrocytes. Front Cell Neurosci 7:179
Bak LK, Walls AB, Schousboe A, Waagepetersen HS (2018) Astrocytic glycogen metabolism in the healthy and diseased brain. J Biol Chem 293:7108–7116
Dringen R, Hoepken H, Minich T, Ruedig C (2007) Pentose phosphate pathway and NADPH metabolism. In: Lajtha A, Gibson GE, Dienel GA (eds) Handbook of neurochemistry and molecular neurobiology: brain energetics integration of molecular and cellular processes. Springer, Berlin, pp 41–62
Takahashi S, Abe T, Gotoh J, Fukuuchi Y (2002) Substrate-dependence of reduction of MTT: a tetrazolium dye differs in cultured astroglia and neurons. Neurochem Int 40:441–448
Wefers H, Komai T, Talalay P, Sies H (1984) Protection against reactive oxygen species by NAD(P)H:quinone reductase induced by the dietary antioxidant butylated hydroxyanisole (BHA): decreased hepatic low-level chemiluminescence during quinone redox cycling. FEBS Lett 169:63–66
Tulpule K, Hohnholt MC, Hirrlinger J, Dringen R (2014) Primary cultures of astrocytes and neurons as model systems to study the metabolism and metabolite export from brain cells. In: Hirrlinger J, Waagepetersen HS (eds) Neuromethods: brain energy metabolism. Springer, New York, pp 45–72
Bisswanger H (2017) Enzyme kinetics: principles and methods. Wiley, Weinheim
Minich T, Yokota S, Dringen R (2003) Cytosolic and mitochondrial isoforms of NADP+-dependent isocitrate dehydrogenases are expressed in cultured rat neurons, astrocytes, oligodendrocytes and microglial cells. J Neurochem 86:605–614
Brandmann M, Nehls U, Dringen R (2013) 8-Hydroxy-efavirenz, the primary metabolite of the antiretroviral drug efavirenz, stimulates the glycolytic flux in cultured rat astrocytes. Neurochem Res 38:2524–2534
Arend C, Ehrke E, Dringen R (2019) Consequences of a metabolic glucose-depletion on the survival and the metabolism of cultured rat astrocytes. Neurochem Res 44:2288–2300
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Balan IS, Fiskum G, Kristian T (2010) Visualization and quantification of NAD(H) in brain sections by a novel histo-enzymatic nitrotetrazolium blue staining technique. Brain Res 1316:112–119
Zhang W, Zhu M, Wang F, Cao D, Ruan JJ, Su W, Ruan BH (2016) Mono-sulfonated tetrazolium salt based NAD(P)H detection reagents suitable for dehydrogenase and real-time cell viability assays. Anal Biochem 509:33–40
Williams-Ashman H, Huggins C (1961) Oxydation of reduced pyridine nucleotides in mammary gland and adipose tissue following treatment with polynuclear hydrocarbons. Pharmacology 4:223–226
Lind C, Cadenas E, Hochstein P, Ernster L (1990) DT-diaphorase: purification, properties, and function. Methods Enzymol 186:287–301
Schultzberg M, Segura-Aouilar J, Lind C (1988) Distribution of DT-diaphorase in the rat brain: biochemical and immunohistochemical studies. Neuroscience 27:763–776
Hollander PM, Ernster L (1975) Studies on the reaction mechanism of DT diaphorase: action of dead-end inhibitors and effects of phospholipids. Arch Biochem Biophys 169:560–567
Ernster L, Danielson L, Ljunggren M (1962) DT diaphorase I. Purification from the soluble fraction of rat-liver cytoplasm, and properties. Biochim Biophys Acta 58:171–188
Park J-S, Lee Y-Y, Kim J, Seo H, Kim H-S (2016) β-Lapachone increases phase II antioxidant enzyme expression via NQO1-AMPK/PI3K-Nrf2/ARE signaling in rat primary astrocytes. Free Radic Biol Med 97:168–178
Liddell JR, Lehtonen S, Duncan C, Keksa-Goldsteine V, Levonen A-L, Goldsteins G, Malm T, White AR, Koistinaho J, Kanninen KM (2016) Pyrrolidine dithiocarbamate activates the Nrf2 pathway in astrocytes. J Neuroinflammation 13:49
Kolossov VL, Ponnuraj N, Beaudoin JN, Leslie MT, Kenis PJ, Gaskins HR (2017) Distinct responses of compartmentalized glutathione redox potentials to pharmacologic quinones targeting NQO1. Biochem Biophys Res Commun 483:680–686
Siegel D, Kepa JK, Ross D (2012) NAD(P)H:quinone oxidoreductase 1 (NQO1) localizes to the mitotic spindle in human cells. PLoS ONE 7:e44861
Bongard RD, Lindemer BJ, Krenz GS, Merker MP (2009) Preferential utilization of NADPH as the endogenous electron donor for NAD(P)H:quinone oxidoreductase 1 (NQO1) in intact pulmonary arterial endothelial cells. Free Radic Biol Med 46:25–32
Pey AL, Megarity CF, Timson DJ (2014) FAD binding overcomes defects in activity and stability displayed by cancer-associated variants of human NQO1. Biochim Biophys Acta 1842:2163–2173
Hosoda S, Nakamura W, Hayashi K (1974) Properties and reaction mechanism of DT diaphorase from rat liver. J Biol Chem 249:6416–6423
Vidal LS, Kelly CL, Mordaka PM, Heap JT (2018) Review of NAD(P)H-dependent oxidoreductases: properties, engineering and application. Biochim Biophys Acta 1866:327–347
Dringen R, Hamprecht B (1998) Glutathione restoration as indicator for cellular metabolism of astroglial cells. Dev Neurosci 20:401–407
Hassinen IE (2018) Signaling and regulation through the NAD+ and NADP+ networks. Antioxid Redox Signal 30:857–874
Blumrich E-M, Kadam R, Dringen R (2016) The protein tyrosine kinase inhibitor tyrphostin 23 strongly accelerates glycolytic lactate production in cultured primary astrocytes. Neurochem Res 41:2607–2618
Dringen R, Gebhardt R, Hamprecht B (1993) Glycogen in astrocytes: possible function as lactate supply for neighboring cells. Brain Res 623:208–214
Vistica DT, Skehan P, Scudiero D, Monks A, Pittman A, Boyd MR (1991) Tetrazolium-based assays for cellular viability: a critical examination of selected parameters affecting formazan production. Cancer Res 51:2515–2520
Patching SG (2017) Glucose transporters at the blood-brain barrier: function, regulation and gateways for drug delivery. Mol Neurobiol 54:1046–1077
Roeder LM, Williams IB, Tildon JT (1985) Glucose transport in astrocytes: regulation by thyroid hormone. J Neurochem 45:1653–1657
Dringen R, Bergbauer K, Wiesinger H, Hamprecht B (1994) Utilization of mannose by astroglial cells. Neurochem Res 19:23–30
Abe K, Saito H (1996) Menadione toxicity in cultured rat cortical astrocytes. Jpn J Pharmacol 72:299–306
Steinmeier J, Dringen R (2019) Exposure of cultured astrocytes to menadione triggers rapid radical formation, glutathione oxidation and Mrp1-mediated export of glutathione disulfide. Neurochem Res 44:1164–1181
Bergbauer K, Dringen R, Verleysdonk S, Gebhardt R, Hamprecht B, Wiesinger H (1996) Studies on fructose metabolism in cultured astroglial cells and control hepatocytes: lack of fructokinase activity and imrrunoreactivity in astrocytes. Dev Neurosci 18:371–379
Dringen R, Hamprecht B (1993) Differences in glycogen metabolism in astroglia-rich primary cultures and sorbitol–selected astroglial cultures derived from mouse brain. Glia 8:143–149
Westhaus A, Blumrich EM, Dringen R (2017) The antidiabetic drug metformin stimulates glycolytic lactate production in cultured primary rat astrocytes. Neurochem Res 42:294–305
Perez-Escuredo J, Van Hee VF, Sboarina M, Falces J, Payen VL, Pellerin L, Sonveaux P (2016) Monocarboxylate transporters in the brain and in cancer. Biochim Biophys Acta 1863:2481–2497
Achanta LB, Rae CD (2017) β-Hydroxybutyrate in the brain: one molecule, multiple mechanisms. Neurochem Res 42:35–49
Dringen R, Peters H, Wiesinger H, Hamprecht B (1995) Lactate transport in cultured glial cells. Dev Neurosci 17:63–69
Wilhelm F, Hirrlinger J (2011) The NAD+/NADH redox state in astrocytes: independent control of the NAD+ and NADH content. J Neurosci Res 89:1956–1964
Liddell JR, Zwingmann C, Schmidt MM, Thiessen A, Leibfritz D, Robinson SR, Dringen R (2009) Sustained hydrogen peroxide stress decreases lactate production by cultured astrocytes. J Neurosci Res 87:2696–2708
Klotz L-O, Hou X, Jacob C (2014) 1,4-naphthoquinones: from oxidative damage to cellular and inter-cellular signaling. Molecules 19:14902–14918
Hollensworth SB, Shen C, Sim JE, Spitz DR, Wilson GL, LeDoux SP (2000) Glial cell type-specific responses to menadione-induced oxidative stress. Free Radic Biol Med 28:1161–1174
Loor G, Kondapalli J, Schriewer JM, Chandel NS, Hoek TLV, Schumacker PT (2010) Menadione triggers cell death through ROS-dependent mechanisms involving PARP activation without requiring apoptosis. Free Radic Biol Med 49:1925–1936
Ying W (2008) NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxid Redox Signal 10:179–206
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Special Issue: In Honor of Prof. Juan Bolanos.
Rights and permissions
About this article
Cite this article
Ehrke, E., Steinmeier, J., Stapelfeldt, K. et al. The Menadione-Mediated WST1 Reduction by Cultured Astrocytes Depends on NQO1 Activity and Cytosolic Glucose Metabolism. Neurochem Res 46, 88–99 (2021). https://doi.org/10.1007/s11064-019-02930-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-019-02930-1