Abstract
Objective
A functional magnetic resonance imaging (fMRI) study was performed during urodynamic examination in healthy adults to determine the responses of functional brain networks to bladder control during urine storage.
Methods
The brain imaging was performed in empty and full bladder states during urodynamic examination. First, we used independent component analysis (ICA) to obtain several resting state network masks, then the brain regions with significantly different regional homogeneity (ReHo) values between the two states were determined using a paired t test (p < 0.05; Gaussian random field correction [GRF]: voxel p < 0.01 and cluster p < 0.05) and presented in their corresponding resting state network (RSN) masks.
Results
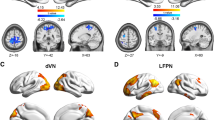
Data sets obtained from the remaining 20 subjects were analyzed after motion correction. Nine RSNs were identified by group-ICA, including the salience network (SN), default mode network (DMN), central executive network (CEN), dorsal attention network (dAN), auditory network (AN), sensorimotor network (SMN), language network (LN), visual network (VN), and cerebellum network (CN). The ReHo values were significantly increased (p < 0.05, GRF corrected) within the SN, DMN, and CEN in the full bladder state compared with the empty bladder state.
Conclusion
Significant changes within the three functional brain networks were demonstrated when the bladder was full, suggesting that SN provides bladder sensation and DMN may provide self-reference, self-reflection, and decision-making about whether to void after assessment of the external environment, while CEN may provide support related to episodic memory, which provides new insight into the processing of bladder control and could serve as a premise to further explore the pathologic process underlying bladder dysfunction.



Similar content being viewed by others
References
Griffiths D (2015) Neural control of micturition in humans: a working model. Nat Rev Urol 12(12):695–705. https://doi.org/10.1038/nrurol.2015.266
Gao Y, Liao L, Blok BFM (2015) A resting-state functional MRI study on central control of storage: brain response provoked by strong desire to void. Int Urol Nephrol 47(6):927–935. https://doi.org/10.1007/s11255-015-0978-0
Blok BF, Willemsen AT, Holstege G (1997) A PET study on brain control of micturition in humans. Brain 120(Pt 1):111–121. https://doi.org/10.1093/brain/120.1.111
Kuhtz-Buschbeck JP, van der Horst C, Pott C, Wolff S, Nabavi A, Jansen O, Junemann KP (2005) Cortical representation of the urge to void: a functional magnetic resonance imaging study. J Urol 174(4 Pt 1):1477–1481. https://doi.org/10.1097/01.ju.0000173007.84102.7c
Matsuura S, Kakizaki H, Mitsui T, Shiga T, Tamaki N, Koyanagi T (2002) Human brain region response to distention or cold stimulation of the bladder: a positron emission tomography study. J Urol 168(5):2035–2039. https://doi.org/10.1097/01.ju.0000027600.26331.11
Zang Y, Jiang T, Lu Y, He Y, Tian L (2004) Regional homogeneity approach to fMRI data analysis. Neuroimage 22(1):394–400. https://doi.org/10.1016/j.neuroimage.2003.12.030
Paakki JJ, Rahko J, Long X, Moilanen I, Tervonen O, Nikkinen J, Starck T, Remes J, Hurtig T, Haapsamo H, Jussila K, Kuusikko-Gauffin S, Mattila ML, Zang Y, Kiviniemi V (2010) Alterations in regional homogeneity of resting-state brain activity in autism spectrum disorders. Brain Res 1321:169–179. https://doi.org/10.1016/j.brainres.2009.12.081
Pamilo S, Malinen S, Hlushchuk Y, Seppa M, Tikka P, Hari R (2012) Functional subdivision of group-ICA results of fMRI data collected during cinema viewing. PLoS ONE 7(7):e42000. https://doi.org/10.1371/journal.pone.0042000
La C, Mossahebi P, Nair VA, Bendlin BB, Birn R, Meyerand ME, Prabhakaran V (2015) Age-related changes in inter-network connectivity by component analysis. Front Aging Neurosci 7:237. https://doi.org/10.3389/fnagi.2015.00237
Erhardt EB, Rachakonda S, Bedrick EJ, Allen EA, Adali T, Calhoun VD (2011) Comparison of multi-subject ICA methods for analysis of fMRI data. Hum Brain Mapp 32(12):2075–2095. https://doi.org/10.1002/hbm.21170
Himberg J, Hyvarinen A, Esposito F (2004) Validating the independent components of neuroimaging time series via clustering and visualization. Neuroimage 22(3):1214–1222. https://doi.org/10.1016/j.neuroimage.2004.03.027
Zou Q, Wu CW, Stein EA, Zang Y, Yang Y (2009) Static and dynamic characteristics of cerebral blood flow during the resting state. Neuroimage 48(3):515–524. https://doi.org/10.1016/j.neuroimage.2009.07.006
Sridharan D, Levitin DJ, Menon V (2008) A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci USA 105(34):12569–12574. https://doi.org/10.1073/pnas.0800005105
Sakakibara R, Hattori T, Yasuda K, Yamanishi T (1996) Micturitional disturbance after acute hemispheric stroke: analysis of the lesion site by CT and MRI. J Neurol Sci 137(1):47–56. https://doi.org/10.1016/0022-510x(95)00322-s
Griffiths D, Derbyshire S, Stenger A, Resnick N (2005) Brain control of normal and overactive bladder. J Urol 174(5):1862–1867. https://doi.org/10.1097/01.ju.0000177450.34451.97
Craig AD (2003) Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol 13(4):500–505. https://doi.org/10.1016/s0959-4388(03)00090-4
Griffiths D, Tadic SD (2008) Bladder control, urgency, and urge incontinence: evidence from functional brain imaging. Neurourol Urodyn 27(6):466–474. https://doi.org/10.1002/nau.20549
Chong JSX, Ng GJP, Lee SC, Zhou J (2017) Salience network connectivity in the insula is associated with individual differences in interoceptive accuracy. Brain Struct Funct 222(4):1635–1644. https://doi.org/10.1007/s00429-016-1297-7
Jarrahi B, Mantini D, Mehnert U, Kollias S (2015) Exploring influence of subliminal interoception on whole-brain functional network connectivity dynamics. In: Conference proceedings: annual international conference of the IEEE engineering in medicine and biology society annual conference, pp 670–674. https://doi.org/10.1109/embc.2015.7318451
Ketai LH, Komesu YM, Dodd AB, Rogers RG, Ling JM, Mayer AR (2016) Urgency urinary incontinence and the interoceptive network: a functional magnetic resonance imaging study. Am J Obstet Gynecol 215(4):449 e441-449 e417. https://doi.org/10.1016/j.ajog.2016.04.056
Kitta T, Chancellor MB, de Groat WC, Shinohara N, Yoshimura N (2016) Role of the anterior cingulate cortex in the control of micturition reflex in a rat model of Parkinson’s disease. J Urol 195(5):1613–1620. https://doi.org/10.1016/j.juro.2015.11.039
Andrew J, Nathan PW (1964) Lesions on the anterior frontal lobes and disturbances of micturition and defaecation. Brain 87:233–262. https://doi.org/10.1093/brain/87.2.233
Blok BF (2002) Central pathways controlling micturition and urinary continence. Urology 59(5 Suppl 1):13–17. https://doi.org/10.1016/s0090-4295(01)01633-8
Raichle ME, Snyder AZ (2007) A default mode of brain function: a brief history of an evolving idea. Neuroimage 37(4):1083–1090. https://doi.org/10.1016/j.neuroimage.2007.02.041
Baumgartner C, Gröppel G, Leutmezer F, Aull-Watschinger S, Pataraia E, Feucht M, Trinka E, Unterberger I, Bauer G (2000) Ictal urinary urge indicates seizure onset in the nondominant temporal lobe. Neurology 55(3):432–434. https://doi.org/10.1212/wnl.55.3.432
Raichle ME (2015) The brain’s default mode network. Annu Rev Neurosci 38:433–447. https://doi.org/10.1146/annurev-neuro-071013-014030
Nardos R, Gregory WT, Krisky C, Newell A, Nardos B, Schlaggar B, Fair DA (2014) Examining mechanisms of brain control of bladder function with resting state functional connectivity MRI. Neurourol Urodyn 33(5):493–501. https://doi.org/10.1002/nau.22458
Bonnici HM, Cheke LG, Green DAE, FitzGerald T, Simons JS (2018) Specifying a causal role for angular gyrus in autobiographical memory. J Neurosci 38(49):10438–10443. https://doi.org/10.1523/JNEUROSCI.1239-18.2018
Jarrahi B, Mantini D, Balsters JH, Michels L, Kessler TM, Mehnert U, Kollias SS (2015) Differential functional brain network connectivity during visceral interoception as revealed by independent component analysis of fMRI TIME-series. Hum Brain Mapp 36(11):4438–4468. https://doi.org/10.1002/hbm.22929
Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD (2007) Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27(9):2349–2356. https://doi.org/10.1523/JNEUROSCI.5587-06.2007
Acknowledgements
The work was supported by the National Natural Scientific Foundation of China (No. 81570688). Thanks to Lingna Zhao for fMRI data collection.
Funding
The study was funded by the by grants from the National Natural Scientific Foundation of China (No. 81570688).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No one declared.
Ethical approval
The study was performed in accordance with the rules laid down in the 1964 Declaration of Helsinki and its later amendments and was approved by the Ethics Committee of China Rehabilitation Research Center (IRB: 2017-002-1).
Informed consent
All subjects have provided informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pang, D., Gao, Y. & Liao, L. Responses of functional brain networks to bladder control in healthy adults: a study using regional homogeneity combined with independent component analysis methods. Int Urol Nephrol 53, 883–891 (2021). https://doi.org/10.1007/s11255-020-02742-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-020-02742-1