Abstract
Titania/silica nanomaterials have many possible applications; however, they can be toxic to living organisms, particularly if the material accumulates in niche environments, e.g. areas colonised by actinomycetes. This study therefore investigated the effect of non-activated and UV light-activated titania/silica nanospheres on an environmental Streptomyces strain. The bacteria were incubated with the nanospheres and subsequently cultured on solid medium. The morphology and elemental composition were analysed using optical and electron microscopy (TEM, STEM) and energy-dispersive X-ray spectroscopy (EDX). The appearance of Streptomyces sp. in the experimental and control samples demonstrated that the nanospheres did not have bactericidal properties in the used dose. Furthermore, the observed strain not only survived in the presence of the nanomaterial but also appeared to play a role in its dissolution with an accumulation of the titanium in the intracellular globules of polyphosphate (volutin). Additionally, it was discovered that the UV light-activated titanium dioxide altered the ability of the bacteria to secrete humic acid. The reported phenomenon might be made possible through an accumulation of titanium in the volutin compounds. These findings suggest that streptomycetes could be employed to participate in the dissolution of nanomaterials which enter the natural environment.
Similar content being viewed by others
1 Introduction
Actinomycetes are Gram-positive bacteria of irregular, cylindrical and filamentous structures which are commonly found in soil, water or manure. They offer great value to human health because they are one of the leading producers of bioactive metabolites, including compounds that have antibacterial, antiviral, antifungal, anticancer and antihelminthic properties. These microorganisms produce many enzymes (cellulases, chitinases, xylanase, hydrolases, etc.) and colourants (mederrhodin, actinorhodin and brown medermycin), and subsequently, actinomycetes play a very important role in the functioning of natural ecosystems, especially those associated with soil, which is where they are primarily isolated. Of the 227 types of actinomycetes, one of the most studied is Streptomyces spp., belonging to the Streptomycetaceae family, and it is this species that is responsible for the production of many commercially important biomolecules (Solecka et al. 2013).
The specific morphology and biological properties of these bacteria determines their enormous adaptability, and this is why they can colonise and survive in a diverse range of environments, including those heavily polluted by human activities. Currently, a new and growing eco-threat is the rapid development of nanotechnology, which could be resulting in an increase in nanomaterials being released into the environment. Depending on the characteristics of the nanomaterials, such as their size, charge, solubility, diffusion, bioavailability and biodegradability, these can be quickly transported via air, water and soil (Hannah and Thompson 2008). Ge et al. (2012) speculate that soil could be the main reservoir of modern nanomaterials released into the environment, which allows them to influence both processes and organisms occurring within the soil. The influence of nanoparticles on living organisms, including soil microorganisms, depends primarily on the interaction at the cellular level. Nanoparticles can induce pore formation in cell walls and penetrate the cell after overcoming the cytoplasmic membrane. They can also access the cells with the help of transporter proteins and ion channels and then bind to intracellular organelles (Navarro et al. 2008).
Knowledge of the technological properties of nanoparticles and their potential toxicity is particularly important in relation to newly produced materials (Borm et al. 2006). This is especially so for the following types of engineered nanoparticles (ENPs), because of their wide range of applications, including nanometals (Au, Ag, Fe) and nanooxides (TiO2, ZnO, ZrO2, CeO2), as well as polymers, surfactants, dyes/pigments, fullerenes and carbon nanostructures (Christian et al. 2008; Ge et al. 2012). Recently, it has been found that some ENPs, for example nanoparticulate titanium dioxide (nano-TiO2) and zinc oxide (nano-ZnO), tend to accumulate in the habitat as a result of their widespread use, e.g. for the production of a variety of commonly used sunscreen formulations and coatings. Nanomaterial presence in the soil has led to a change in the recorded composition of the soil bacterial community, where the observed effects increase with dose (Ge et al. 2012). Furthermore, some photoactive nanomaterials have successfully been used as antibacterial photoactivated agents, and these include mesoporous silica nanospheres and mesoporous silica nanotubes modified with nanocrystalline titanium dioxide placed in the pores. These nanocomposites showed strong bactericidal activity resulting in the penetration of cell guards, internalisation and inhibition of cell proliferation (Cendrowski et al. 2013; Cendrowski et al. 2014b). Moreover, other studies have shown that the antibacterial effect of nanomaterials increases with a decrease of their size (Paredes et al. 2014). This new, forward-looking method for eliminating microbiological hazards could be a major reason for the wider use of nanomaterials with proven antibacterial activity (e.g. for water purification systems) and thus could cause an increase in their accumulation in and impact on the environment. In 2012, the official worldwide production of silica and titanium dioxide nanomaterials was estimated to around 10,000 t of each of them, and the amount of produced nanostructures increases every year (Piccinno et al. 2012). Therefore, nanomaterials may be considered as emerging pollutants. Nevertheless, current knowledge about the possible effects of different nanoparticles on living organisms continues to be insufficient, in particular the difficulties in predicting the distant consequences of exposure to these nanoparticles. Therefore, the main goal of the current work is to determine if (and how) titania-modified silica nanospheres influence the cell of actinomycetes of the genus Streptomyces. This study attempts to determine the possible role that certain commonly found soil bacteria play in the process of dissolution of nanomaterials from their natural environment.
2 Materials and Methods
2.1 Characteristics and Culture Conditions of Streptomyces sp.
An environmental isolate—Streptomyces sp. Modena/2012—was obtained from the Department of Immunology, Microbiology and Physiological Chemistry, West Pomeranian University of Technology, Szczecin, Poland, and was used as the biological material. The genus was confirmed by characteristic morphological hallmarks according to the Bergey’s Manual of Systematic Microbiology (2012) and an analysis of the 16S rDNA gene fragment (100 % similarity to the NCBI record ID: KP126332). Sequencing of the amplicon was conducted at the Institute of Biochemistry and Biophysics of the Polish Academy of Sciences. The isolate used in this study possessed a chromatographically confirmed ability to produce humic acid.
2.2 Preparation of Pure Culture of Streptomyces sp.
The bacterial strain used in the research was initially grown on starch casein agar medium (Küster and Williams 1964) (g/L: soluble starch, 10; casein, 0.3; KNO3, 2; MgSO4, 0.05; CaCO3, 0.02; FeSO4, 0.01; NaCl, 2; K2HPO4, 2; agar, 20). Inoculum for liquid cultures was prepared by collecting spores from solid medium using an inoculation loop and suspending in 1 mL of brain heart infusion broth (BHI, Oxoid). Afterwards, the inoculum was thoroughly vortexed. Then, 10 μL was removed and added to 990 μL of BHI medium, which had been previously poured into sterile tubes (1.5 μL; Eppendorf). The cultures were maintained for 7 days at 28 °C and without access to light.
2.3 Synthesis of Solid Silica Nanospheres Coated by the Mesoporous Shell (mSiO2/SiO2)
Solid silica nanospheres were prepared according to modified Stöber sol–gel process as previously described (Cendrowski et al. 2013). Briefly, 2.5 mL of ammonium (NH3·H2O) and 50.0 mL of ethanol (EtOH) were mixed in a reactor and refluxed by magnetic stirring. At a temperature of 60 °C, 1.5 mL of the tetraethyl orthosilicate (TEOS) was added before the suspension was stirred for 24 h. Finally, the solvents were evaporated leaving a dried powder of silica nanospheres.
To create a mesoporous external layer on the silica spheres, a mixture of 160 mg hexadecyl(trimethyl)azanium bromide (CTAB), 0.48 mL NH3·H2O, 25 mL EtOH, 30 mL H2O and silica nanospheres was prepared, according to the procedure described previously (Cendrowski et al. 2013). After 30 min of stirring, 0.28 mL TEOS was added and the mixture was stirred further for 24 h. The suspension was then centrifuged at 5900×g for 10 min before the silica nanospheres were thoroughly washed with ethanol. In order to remove the surfactant, the sample was annealed in the air at 600 °C for 3 h and the core/shell structure with solid core and mesoporous shell was obtained (mSiO2/SiO2).
2.4 Synthesis of Titania-Modified Silica Nanospheres (TiO2/SiO2)
The following process was used to functionalize the mesoporous silica shell of the spheres with titanium dioxide. One hundred milligrammes of silica nanostructures were dispersed in 2 mL of concentrated titanium(IV) butoxide (TBT). Next, the suspension was sonicated for 3 h in anhydrous conditions at 45 °C. The suspension was then diluted with propanol and centrifuged (5900×g for 10 min) to separate the excess titanium dioxide precursor. In order to remove the excess TBT, the sample was washed several times with propanol. After the purification step, the nanostructures filled with TBT were treated with ethanol to hydrolyse the precursor to the titanium dioxide. Finally, the sample was evaporated and heated in an air flow at 600 °C for 4 h to transform the titanium dioxide into the anatase phase.
2.5 Activation of the Titanium Dioxide in TiO2/SiO2
The pre-activation of the TiO2/SiO2 was carried out in the inner-irradiation-type reactor with a mercury lamp of 150 W as a light source (cooled by the inner water jacket). For the pre-activation, TiO2/SiO2 was dispersed in distilled water, with an initial concentration of 1 mg/mL, and placed in the photoreactor. The suspension was continuously stirred during the experiment. The nanocatalyst was irradiated for the defined times of 5, 15, 30 and 60 min. After titanium dioxide pre-activation, the 200 μL of the catalyst suspension was collected for the in vitro studies described in Section 2.6.
2.6 The In Vitro Treatment of Titanium/Silica Nanospheres on Streptomyces sp.
The contact test was prepared by adding 200 μL of nanomaterial (in a concentration of 1 mg/mL) to 1 mL of Streptomyces sp. liquid culture. The nanomaterial was either pre-activated by UV light or non-activated (see Section 2.5). The used dose has previously shown a bactericidal effect against Escherichia coli (Cendrowski et al. 2013). The irradiated nanospheres were collected after 5, 10, 30 and 60 min, respectively, and promptly transferred to bacterial cultures. For the control samples, 200 μL of sterile water was added instead of the nanomaterial. After 24 h of incubation, bacteria were inoculated to starch casein agar and incubated for 7 days at 28 °C without any access to light. The growth of bacteria was characterised using a stereoscopic microscope, whereas the Streptomyces cells were observed in detail using a high-resolution transmission electron microscopy (HR-TEM).
An alamarBlue® toxicity assay was performed in order to measure viability of the chosen streptomycete after contact with five doses of nanomaterial ranging from 0 up to 0.1 mg/mL. The experiments were conducted in 96-well plates. Twenty microlitres of nanomaterials irradiated with the UV light (as shown in Section 2.5) was transferred into wells. Afterwards, the wells were filled with 178 μL of BHI broth and inoculated with 2 μL of the spore suspension. Each well was filled with the biological material from the same suspension. Cultures were kept in 28 °C for 48 h. After the incubation, 20 μL of alamarBlue® reagent was transferred to the wells and incubated for 4 h. The readings were taken after 15, 45, 105, 165 and 240 min according to the user’s manual provided by the producer. The absorbance was measured at wavelengths of 570 and 600 nm. Each sample was prepared in six repetitions. Similarly, six controls without bacteria were prepared for every dose. Silica mesoporous nanospheres and titanium dioxide alone were used as additional controls for the comparison of the results.
2.7 Characterisation Techniques, Statistical Analysis and Reproducibility of the Results
TEM images were collected using an FEI Tecnai G2 F20 S-Twin with an accelerating voltage of 200 kV, and X-ray dispersion spectroscopy (EDX) was employed. Specific surface area of the samples was measured through adsorption N2 isotherm using the (interpreted with the Brunauer-Emmett-Teller or BET model) Quadrosorb SI (Quantachrome Instruments). Crystallographic phase identification was performed using X’Pert Philips PRO X-ray diffractometer (X’Pert PRO Philips diffractometer, CoKa radiation). Secretion of humic acid was confirmed by crystallographic phase identification, using X’Pert Philips PRO X-ray diffractometer (X’Pert PRO Philips diffractometer, CoKa radiation). Metabolic tests regarding determination of viability of the used actinomycete were analysed with the use of analysis of variance (ANOVA) and the Student’s t test, at the significance level of p = 0.05. Experiments in which the outcome was an observation had been repeated three times.
3 Results
3.1 Nanomaterial Characterisation
In Fig. 1, the morphology of the silica nanostructures before and after titania modification is demonstrated. The HR-TEM micrograph (Fig. 1a) clearly shows that the mesoporous silica nanospheres are composed of a solid core with porous shell on its surface. The average thickness of the porous shell is ∼30 nm. Dominant diameter of the obtained nanospheres ranges from 160 to 190 nm (ESM 1). The TEM image presented in Fig. 1b reveals the changes in the structure of the mesoporous silica shell after the functionalization with titanium dioxide. The contrast between the solid core and the porous shell is not as clear after the deposition of titania. The diameter of the spheres has not significantly changed, meaning that the majority of titania is inside the pores of the mesoporous silica structure. In the EDX spectrum showing the titania-modified sample, the peak assigned to titanium is detected. The elemental mapping of TiO2/SiO2 shows titanium, silica and oxygen as the only elements present in the sample. The mapping was performed on the nanoobject and is shown in Fig. 1c.
3.2 The Bactericidal Effect of Mesoporous Silica Nanospheres with Titanium Dioxide in Relation to Streptomyces Cells
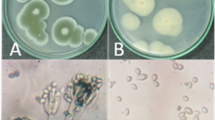
On the basis of the stereoscopic microscope analysis, there did not appear to be any bactericidal effect of the tested dose of TiO2/SiO2 nor any changes in morphology of the bacterial colonies, in comparison to control plates. The TEM microscopic analysis of the Streptomyces cells presented in the Fig. 2a–c shows the hyphae and exospore form of the bacteria. The exospore structure (attached and free) is darker than the hyphae due to the accumulated phosphate (Fig. 2a, b). An example of the pseudomycelium containing deposits of phosphate is presented in Fig. 2c. Electron microscopic analysis of the Streptomyces cells after a 24-h incubation with TiO2/SiO2 also shows bacteria in the hyphae and exospore form (Fig. 2d–f). There were no significant signs of deformation of the exospore form following exposure to the nanospheres. The intracellular structure of the exospore presented in Fig. 2e has a regular shape which is caused by the phosphate deposit formation in the cluster form. Single cases of the nanosphere presence in the sample were also observed.
From the comparison of the microscopic images after incubation with and without mesoporous silica nanospheres with titanium dioxide (Fig. 2), there was no significant difference in Streptomyces shape, structure integrity or additional elements present, between the observed samples.
The alamarBlue® toxicity assay did not give a statistically relevant difference between controls without nanomaterials and the experimental samples. Moreover, no differences were obtained between silica and titanium dioxide alone and the nanocomposite as well. All cultures showed around 50 % reduction of the reagent after 4 h of incubation regardless of the used nanomaterial.
In order to determine the integrity and chemical composition of the bacterial cells after the incubation with nanospheres, energy-dispersive X-ray spectroscopy (TEM mode) was used. Exemplary data from the elemental composition of the Streptomyces cells is provided in Fig. 3. Other cells analysed in the study also presented such composition. The scanning transmission electron (STEM) microscopic image in Fig. 3 shows the bacteria in the hyphae form (the red square indicates the area analysed in the elemental mapping). The signal from the carbon material (after removing background signal from carbon film on the TEM grid) overlaps with the Streptomyces cells. The carbon signal overlaps with the signals from phosphorus and oxygen. The simultaneous presence of oxygen and phosphorus confirms that the TEM identification of the darker agglomerates (in the bacteria cells) is polyphosphates. The presence of titanium in the inert bacteria structure overlaps with the phosphorus and oxygen signal source. The same position of phosphorus and titanium suggests binding of titanium dioxide by phosphorous particles in the intracellular structure of the Streptomyces. The signal coming from the silicon particles is distributed across the analysed area and does not overlap with any particular part of the bacterial cell. A number of factors suggest that there has been a dissolution of the nanostructures, regardless of whether the nanomaterial had been exposed to UV light or not. These factors included an incoherent distribution of the silicon particles, the presence of titanium dioxide in the deposit of polyphosphate and the difficulty in being able to identify the presence of the nanospheres.
Elemental mapping of the Streptomyces cells with internalised titanium dioxide from mesoporous silica nanospheres. Scanning transmission electron microscopic image (STEM) with marked red square shows analysed part of the bacteria. In the following image of the elemental distribution, signal from individual elements are marked with specific colour: carbon—red (C), oxygen—orange (O), silicon—yellow (Si), phosphorus—green (P) and titanium—blue (Ti)
Mesoporous silica nanospheres modified with titanium dioxide after a 24-h incubation with Streptomyces cells were studied in order to determine the stability of the nanostructures. The nanospheres after the incubation were analysed with transmission electron microscope (TEM) and energy-dispersive X-ray spectroscopy (EDX) showing nanospheres structure and elemental composition (Fig. 4). A comparison of the image in Fig. 4a–c shows a noticeable difference in the structure of the silica nanospheres functionalized with titanium dioxide. TEM images in Fig. 4b, c clearly indicate that the mesoporous silica shell is no longer observed. Additionally, on the surface of the nanospheres, there has been an appearance of agglomerates (indicated in the image as darker spots). The agglomerates could be assigned to the released TiO2 from the mesoporous shells induced during the incubation process. The presence of TiO2 in the nanospheres was confirmed using the EDX spectrum (Fig. 2d). As well as the presence of titanium and oxygen (marked in the spectrum as Ti and O), there are peaks attributed to sodium—Na, phosphate—P, potassium—K (from the microelements contained in the culture medium) and copper—Cu (from the TEM grid) that were detected.
3.3 Streptomyces Cells Stimulation with Internalised Titanium Dioxide from the Mesoporous Silica Nanospheres (mSiO2/TiO2)
The bacterial growth after 24 h of contact with the nanomaterial activated by UV light showed similar intensity to the control plates. The morphology of the formed pseudomycelium did not show a significant difference. Nevertheless, a gradual change of colour of the pseudomycelium was observed. The most intensive hue was obtained in the culture after the incubation with the nanospheres that had been activated for 60 min (Fig. 5a–e). The darkening of the medium was associated with the appearance of humic acid. The ability to secrete such compounds had been observed previously for Streptomyces sp., although it occurred normally after over 14 days for the culture.
Additionally, the sample analysed by the UV–Vis spectroscopy revealed an increase in intensity (during activation time) at a peak of 428.5 nm. The measured peak intensity was compared to the distilled water spectrum in order to distinguish between the unimportant backgrounds. This peak was assigned to the exopolymeric substances secretion (humic acid) by the bacteria. The spectrum of the analysed samples is presented in Fig. 6a. The intensity of the peak is measured at 428.5 nm and is shown in Fig. 6b. From the analysis of the peak intensity, there is a noticeable increase in the secretion of exopolymeric substances after contact with the activated titanium dioxide. The UV–Vis spectroscopy analysis correlates with the stereoscopic microscope observation of pseudomycelium after culture on starch casein agar. The presence of humic acid was also confirmed by electron microscopy and the XRD (ESM 1).
4 Discussion
The large-scale use of nanomaterials in industry causes their accumulation in the natural environment. Titanium dioxide is often used as a component in manufacturing processes, for example in food and personal care products. It can be noxious to living organisms, and so, there is a need to evaluate the potential impact of this nanomaterial on biocenosis and, above all, to determine its fate in the natural environment (Borm et al. 2006; Kunzmann et al. 2011; Weir et al. 2012). Results of this study, especially culture tests, suggest that the subject microorganism can coexist with the nanomaterial in tested conditions (in vitro) without relevant changes to its morphology. Also, the growth intensity was comparable on both experimental and control plates, which shows that the used dose of titania/silica nanospheres, which can have bactericidal effects, did not influence the viability of tested Streptomyces sp. Furthermore, the appearance of numerous observed spores (Fig. 3e) implies the readiness of those cells to develop into new pseudomycelium. This suggests that the preceding addition of nanomaterial to the liquid culture did not cause an inhibition of mechanisms leading to spore development. These results are in agreement with Ge et al. (2012) who found no toxicity of titanium dioxide (including the dose used in our study) on other strains belonging to the Streptomyces genus. Moreover, they showed that the quantity of those microorganisms increased during the experiment. These findings suggest that the bacterial strain used in our experiments may be biocompatible with the titania/silica nanospheres at the applied doses.
The microorganism was not only able to survive under the experimental conditions but it also absorbed titania from the used nanomaterial. In the cytosol of actinomycetes cells, including the Streptomyces genus, inclusions of polyphosphate globules (of various sizes) are present which are also defined as volutin (Fig. 3, STEM). Volutin provides bacterial cells with a source of energy and inorganic phosphorous, while also acting as chelator of various elements such as calcium and magnesium (Achbergerová and Nahálka 2011; Liu et al. 2013). The titanium in the form of ions was not expected in our experimental conditions, although due to the electrostatic properties of polyphosphate, it may be assumed that this substance, gathered in the Streptomyces sp. cells, could work as an adsorbent of titanium dioxide presented in our study.
The appearance of the nanospheres after the incubation with bacteria and the changed elemental composition (Fig. 4d, e) may suggest a dissolution of the nanomaterial, which implies that Streptomyces sp. possess the ability to change the structure of this compound under conditions which are optimal for the bacterial growth. This is a considerable feat because nanospheres have high thermal stability even at temperatures reaching 1000 °C and their durability under conditions occurring in soils (Dong-Wook et al. 2005). The described phenomenon offers a potential novel method for removing nanomaterials after they have entered into the ecosystems, which could potentially be performed by environmental microorganisms, e.g. Streptomyces sp. Moreover, this may provide a new view to forecasting the fate of nanomaterials released in natural environments, particularly because direct bacterial dissolution of titania/silica nanospheres has not been described before. The nanostructures removal is also significant in terms of the industrial production of such materials, which eventually get into ecosystems (Hannah and Thompson 2008; Weir et al. 2012). Microbial ability to perform decomposition processes of the nanomaterials may be a solution to an increasing amount of “nanogarbage” in the natural environments.
Previously, it has been shown that nanosilica is biodegradable under the simulated conditions of living organisms (Cauda et al. 2010), but evidence involving bacteria in the process of biodegradation have not been published to date. However, silica nanospheres were degraded when placed in a simulated body fluid (SBF) and incubated at a temperature of 37 °C, as confirmed by TEM images and BET results. The significant changes in the specific surface area and pore volume were attributed to partial hydrolysis and dissolution of the material. The changes in the structure of the nanosphere have also been shown using TEM images, which clearly indicate that the edges of the nanostructures were blurred. Finnie et al. (2009) investigated the degradation of silica microparticles in phosphate-buffered saline (at temperature 37 °C). The results of their research showed that the dissolution rate was linear and grew with the increased surface area of the sample. Silica can hydrolyse to form silica acid, Si(OH)4, in the environment imitating living organisms. Silica acid from the degradation of silica gel granules was found to diffuse through the blood stream and lymph and further excreted with the urine. The rate of silicic acid extraction was 1.8 mg silicone per day (Lai et al. 1998). Moreover, nanosilica degradation is pH dependent, as shown by Hu et al. (2010), where nanotubes were resistant to degradation at pH 1, dissolution rose slightly at pH 3, while the silica nanotubes solubility increased rapidly at pH ≥5. A rapid dissolution in the initial stage (6 h) reached over 80 % at pH 8, followed by a slow degradation to nearly 100 % after 12 h (Hu et al. 2010). When cultured in media, used in this study, streptomycetes generally altered the pH to acidic. According to the preceding arguments, it is unlikely that the dissolution of nanomaterial was caused by changes to the acidity of the environment.
The literature does not consist many information on the titanium dioxide dissolution. According to Ziemniak et al. (1993), in the titanium dioxide species under the considered pH and temperature, the equilibrium of the solubility is found in the range of micro- to nanomoles per litre (Ziemniak et al. 1993; Knauss et al. 2001). Schmidt and Vogelsberger (2006) studied the dissolution kinetics of titanium dioxide nanoparticles in the NaCl solutions at temperatures of 25 and 37 °C. According to them, the solubility of titanium dioxide depends strongly on the pH (increased in the acidic region pH <3) of the dissolution milieu and on the morphology of the titanium dioxide. The saturation concentration is not stable without an electrostatic or chemical stabilisation. In the frame of our investigation, titanium dioxide will show similar instability to the dissolved silica. Titanium dioxide is introduced to the mesopores of silica nanospheres in a small percentage and its particles can be dissolved in the medium containing NaCl (e.g. PBS and culture medium) (Schmidt and Vogelsberger 2006). These arguments support the hypothesis that titanium dioxide, detected in the bacterial cells, was detached from the nanocomposite during dissolution of the silica nanospheres and subsequently dissolved to the state in which it was available to the microorganism (Schmidt and Vogelsberger 2006; Li et al. 2007; Cauda et al. 2010; Yamada et al. 2012).
Titanium dioxide may show toxicity against microbial cells after the irradiated by UV light. This aspect was confirmed in previous studies on E. coli using the same dosage as in our experiments (Cendrowski et al. 2013). The bactericidal properties can be successfully used for the production of antimicrobial layers, but for the same reason, this may result in the release of noxious compounds into the natural environment (Hannah and Thompson 2008; Kunzmann et al. 2011). Our study shows that the activated by elucidation nanospheres did not affect the viability of the bacteria (regarding applied doses and used experimental set) while at the same time did alter the ability of the humic acid compounds secretion, what may lead to equivocal implications. For Streptomyces spp. the secretion of secondary metabolites is often associated with a release of bioactive compounds such as actinorhodin which shows an antimicrobial activity against Gram-positive bacteria (Palanichamy et al. 2011). The underlying mechanism of increased pigmentation may be associated with another feature of pigments produced by streptomycetes. Stankovic et al. (2012) indicated that such substances may have an antioxidative properties. Activation of TiO2 causes an increase of ROS number in the environment. This suggests that secretion of pigment is a defensive behaviour in studied bacteria, which may explain why we did not observe a relevant decrease in viability. The fact that we observed the pigmentation only after irradiation of nanomaterial is important because it may help in forecasting the possible bacterial behaviour and an influence of the TiO2 on the environmental streptomycetes in relation to the day/night shift. Petković et al. (2011) also studied the cytotoxicity of the ROS produced by the pre-activeted titanium dioxide. They found that irrespective to the particle size, photoactivated anatase TiO2 particles retained elevated reactivity even after the termination of UV exposure (Petković et al. 2011). Thus, our results are in accordance with these studies. On the other hand, the difference is that due to the absence of the cell wall in the HepG2 cells and inability to a promptly secrete antioxidants, these cells were being damaged. Bacterial cells with resistant cell wall and the tendency to produce pigments and other metabolites are capable of neutralising reactive oxygen species. Streptomycetes are known for active secondary metabolism which produces high amounts of ROS (Stankovic et al. 2012). For that reason, these bacteria evolutionally developed defensive mechanisms against these factors such as HbpS-SenS-SenR redox sensing system from Streptomyces reticuli described by Ortiz and Groves (2009). Therefore, we assume that longer irradiation time resulted in the increased amount of ROS, eventually leading to higher secretion of pigment as a stress response.
Maurer-Jones et al. (2013) reported an increase in the secretion of secondary metabolites (flavins) as a reaction to contact with TiO2, which has been demonstrated using Shewanella oneidensis. This can have a positive impact on industry in the context of obtaining greater quantity of the desired product from bioreactors. On the flip side, it can have a negative impact on the natural environment by reducing the soil microbiota diversity caused by the forced release of antibiotic substances into the natural habitat. This hypothesis may correspond with results published by Ge et al. (2012) who showed that the quantity of streptomycetes increased after treatment with TiO2 nanoparticles as opposed to other taxa. Therefore, even if titanium dioxide does not show direct bactericidal effect against the natural soil bacterial communities, it may possibly have an indirect influence by inducing an unnatural secretion of antibiotics. Nevertheless, this statement needs to be thoroughly investigated in further research.
TiO2/SiO2 nanospheres and other nanocomposites made from silica and titanium dioxide in various shapes and sizes (nanotubes, nanoflakes, shapeless particles and agglomerates, nano- and micrometre-size structures) have been studied extensively in the laboratory scale for the last decade (Li et al. 2005; Cendrowski et al. 2011; Oguma et al. 2013; Liu et al. 2015). Due to their chemical and physical properties, they have a great potential for the photocatalytic and biological applications. Imamura et al. had already published the synthesis and application of the nanocomposites from titanium dioxide and silica, for the organic compounds decomposition, in 1989 (Imamura et al. 1989). These types of nanocomposites are studied for the water purification and for the self-cleaning surface coatings. Nevertheless, the release of this type of nanostructures to the environment may have an opposite effect to the intended. Additionally, observed stimulation of the bacteria with pre-activated TiO2/SiO2 nanospheres should be (in the future) considered also for the others types of the titanium dioxide nanocomposites that have potential application as a photocatalyst (regarding potential risk of uncontrolled release to the environment) or in the self-cleaning surface coatings (Tung and Daoud 2009; Linley et al. 2014; Cendrowski et al. 2014a).
The reaction between titanium and volutin granules might have caused changes in the trans-membranous transport system of the cells which subsequently led to the increased humic acid secretion. The interaction did not reduce viability of the experimental strain, which may be associated with the biological characteristics of polyphosphate. Negoda et al. (2009) described that this polymer in the cells of Streptomyces lividans has an influence on the activity of potassium channels (KcsA) as volutin acts as a polyanion which is possibly an integral part of those channels and selectively reacts with cations. We suggest that the acceleration of the humic acid secretion might have been caused by the change in the movement of elements between the cell and environment or other changes in the bacterial metabolic activity which were connected with the internalisation of titanium in the intracellular polyphosphate compounds. Nevertheless, further tests are planned to describe the interactions more broadly and to elucidate the effect of different nanomaterial doses and the secretion of secondary metabolites by Streptomyces sp.
5 Conclusions
In conclusion, the present results of this study suggest that the Streptomyces cells may be able to actively participate in the processes of dissolution of titania/silica nanospheres and their bioavailability, regardless of whether activated by the UV light or not. This effect seems to be generally neutral for these actinomycetes, although the used nanomaterial may probably determine new and effective external factors which promote their growth. Furthermore, the nanomaterial, when activated by UV light, accelerates humic acid secretion in these bacteria, which may possibly alter interactions between bacteria in the soil, if connected with a secretion of secondary metabolites such as antibiotics (which was not studied in this research). The hypothetical effect of the UV light-activated nanospheres on Streptomyces sp. cells is presented in Fig. 7. This study is the first to describe observed interactions between Streptomyces and titania/silica nanomaterials. Further studies are required to explain the molecular basics of the discovered phenomena.
References
Achbergerová, L., Nahálka, J. (2011). Polyphosphate—an ancient energy source and active metabolic regulator. Microbial Cell Factories 10. doi: 10.1186/1475-2859-10-63
Borm, P. J. A., Robbins, D., Haubold, S., Kuhibusch, T., Fissan, H., Donaldson, K., Schins, R., Stone, V., Kreyling, W., Lademann, J., Hartmann, J., Warheit, D., & Oberdorfer, J. (2006). The potential risk of nanomaterials: a review carried out for ECETOC. Particle and Fibre Toxicology, 3, 1–25. doi:10.1186/1743-8977-3-11.
Cauda, V., Schlossbauer, A., & Bein, T. (2010). Bio-degradation study of colloidal mesoporous silica nanoparticles: effect of surface functionalization with organo-silanes and poly(ethylene glycol). Microporous and Mesoporous Materials, 132, 60–71. doi:10.1016/j.micromeso.2009.11.015.
Cendrowski, K., Chen, X., Zielinska, B., Kalenczuk, R. J., Rümmeli, M. H., Büchner, B., Klingeler, R., & Borowiak-Palen, E. (2011). Synthesis, characterization, and photocatalytic properties of core/shell mesoporous silica nanospheres supporting nanocrystalline titania. Journal of Nanoparticle Research, 13, 5899–5908. doi:10.1007/s11051-011-0307-1.
Cendrowski, K., Peruzynska, M., Markowska-Szczupak, A., Chen, X., Wajda, A., Lapczuk, J., Kurzawski, M., Kalenczuk, R., Drozdzik, M., Mijowska, E. (2013) Mesoporous silica nanospheres functionalized by TiO2 as a photoactive antibacterial agent. Journal of Nanomedicine Nanotechnology 4. doi: 10.4172/2157-7439.1000182.
Cendrowski, K., Jedrzejczak, M., Dybus, A., Peruzynska, M., Drozdzik, M., & Mijowska, E. (2014a). Preliminary study towards enhancement of photoactivity performance using biocompatible titanium dioxide/carbon nanotubes composite. Journal of Alloys and Compounds, 605(25), 173–178. doi:10.1016/j.jallcom.2014.03.112.
Cendrowski, K., Peruzynska, M., Markowska-Szczupak, A., Chen, X., Wajda, A., Lapczuk, J., Kurzawski, M., Kalenczuk, R. J., Drozdzik, M., & Mijowska, E. (2014b). Antibacterial performance of nanocrystallined titania confined in mesoporous silica nanotubes. Biomedical Microdevices, 16, 449–458. doi:10.1007/s10544-014-9847-3.
Christian, P., von der Kammer, F., Baalousha, M., & Hofmann, T. (2008). Nanoparticles: structure, properties, preparation and behaviour in environmental media. Ecotoxicology, 17, 326–343. doi:10.1007/s10646-008-0213-1.
Dong-Wook, L., Son-Ki, I., & Kew-Ho, L. (2005). Mesostructure control using a titania-coated silica nanosphere framework with extremely high thermal stability. Chemistry of Materials, 17, 4461–4467. doi:10.1021/cm050485w.
Finnie, K. S., Waller, D. J., Perret, F. L., Krause-Heuer, A. M., Lin, H. Q., Hanna, J. V., & Barbe, C. J. (2009). Biodegradability of sol–gel silica microparticles for drug delivery. Journal of Sol-Gel Science and Technology, 49, 12–18. doi:10.1007/s10971-008-1847-4.
Ge, Y., Schimel, J. P., & Holden, P. A. (2012). Identification of soil bacteria susceptible to TiO2 and ZnO nanoparticles. Applied and Environmental Microbiology, 78, 6749–6758. doi:10.1128/AEM.00941-12.
Hannah, W., & Thompson, P. B. (2008). Nanotechnology risk and the environment: a review. Journal of Environmental Monitoring, 10, 291–300. doi:10.1039/b718127m.
Hu, K. W., Hsu, K. C., & Yeh, C. S. (2010). pH-dependent biodegradable silica nanotubes derived from Gd(OH)3 nanorods and their potential for oral drug delivery and MR imaging. Biomaterials, 31, 6843–6848. doi:10.1016/j.biomaterials.2010.05.046.
Imamura, S., Tarumoto, H., & Ishida, S. (1989). Decomposition of 1,2-dichloroethane on titanium dioxide/silica. Industrial & Engineering Chemistry Research, 28(10), 1449–1452. doi:10.1021/ie00094a001.
Knauss, K. G., Dibley, M. J., Bourcier, W. L., & Shaw, H. F. (2001). Ti(IV) hydrolysis constants derived from rutile solubility measurements made from 100 to 300 C. Applied Geochemistry, 16, 1115–1128. doi:10.1016/S0883-2927(00)00081-0.
Kunzmann, A., Andersson, B., Thurnherr, T., Krug, H., Scheynius, A., & Fadeel, B. (2011). Toxicology of engineered nanomaterials: focus on biocompatibility, biodistribution and biodegradation. Biochimica et Biophysica Acta, 1810, 361–373. doi:10.1016/j.bbagen.2010.04.007.
Küster, E., & Williams, S. T. (1964). Selection of media for isolation of Streptomycetes. Nature, 202, 928–929. doi:10.1038/202928a0.
Lai, W., Ducheyne, P., Garino, J. (1998). Removal pathway of silicon released from bioactive glass granules in vivo. In: R.Z. LeGeros, L. LeGeros, (Ed.) (pp. 383–386). Bioceramics. World Scientific Publishing Co., Singapore.
Li, X., Zhang, L., Dong, X., Liang, J., & Shi, J. (2007). Preparation of mesoporous calcium doped silica spheres with narrow size dispersion and their drug loading and degradation behaviour. Journal of Microporous and Mesoporous Materials, 102, 151–158. doi:10.1016/j.micromeso.2006.12.048.
Li, Z., Hou, B., Xu, Y., Wu, D., & Sun, Y. (2005). Hydrothermal synthesis, characterization, and photocatalytic performance of silica-modified titanium dioxide nanoparticles. Journal of Colloid and Interface Science, 288, 149–154. doi:10.1016/j.jcis.2005.02.082.
Linley, S., Liu, Y. Y., Ptacek, C. J., Blowes, D. W., & Gu, F. X. (2014). Recyclable graphene oxide-supported titanium dioxide photocatalysts with tunable properties. ACS Applied Materials & Interfaces, 6, 4658–4668. doi:10.1021/am4039272.
Liu, H., Deng, L., Sun, C., Li, J., & Zhu, Z. (2015). Titanium dioxide encapsulation of supported Ag nanoparticles on the porous silica bead for increased photocatalytic activity. Applied Surface Science, 326, 82–90. doi:10.1016/j.apsusc.2014.11.110.
Liu, K., Lin, X., & Zhao, J. (2013). Toxic effects of the interaction of titanium dioxide nanoparticles with chemicals or physical factors. International Journal of Nanomedicine, 8, 2509–2520. doi:10.2147/IJN.S46919.
Maurer-Jones, M. A., Gunsolus, I. L., Meyer, B. M., Christenson, C. J., & Haynes, C. L. (2013). Impact of TiO2 nanoparticles on growth, biofilm formation, and flavin secretion in Shewanella oneidensis. Analytical Chemistry, 85, 5810–5818. doi:10.1021/ac400486u.
Navarro, E., Baun, A., Behra, R., Hartmann, N. B., Filser, J., Miao, A. J., Quigg, A., Santschi, P. H., & Sigg, L. (2008). Environmental behaviour and ecotoxicity of engineered nanoparticles to algae, plants and fungi. Ecotoxicology, 17, 372–386. doi:10.1007/s10646-008-0214-0.
Negoda, A., Negoda, E., Xian, M., & Reusch, R. N. (2009). Role of polyphosphate in regulation of the Streptomyces lividans KcsA channel. Biochimica et Biophysica Acta, 1788, 608–614. doi:10.1016/j.bbamem.2008.12.017.
Oguma, J., Kakuma, Y., Murayama, S., & Nosaka, Y. (2013). Effects of silica coating on photocatalytic reactions of anatase titanium dioxide studied by quantitative detection of reactive oxygen species. Applied Catalysis B: Environmental, 129(17), 282–286. doi:10.1016/j.apcatb.2012.09.034.
Ortiz, D., & Groves, M. R. (2009). The three-component signalling system HbpS–SenS–SenR as an example of a redox sensing pathway in bacteria. Amino Acids, 37, 479–486.
Palanichamy, V., Hundet, A., Mitra, B., & Reddy, N. (2011). Optimization of cultivation parameters for growth and pigment production by Streptomyces spp. isolated from marine sediment and rhizosphere soil. The International Journal of Plant, Animal and Environmental Sciences, 1, 158–170.
Paredes, D., Ortiz, C., & Torres, R. (2014). Synthesis, characterization, and evaluation of antibacterial effect of Ag nanoparticles against Escherichia coli O157:H7 and methicillin-resistant Staphylococcus aureus (MRSA). International Journal of Nanomedicine, 9, 1717–1729. doi:10.2147/IJN.S57156.
Petković, J., Küzma, T., Rade, K., Novak, S., & Filipi, M. (2011). Pre-irradiation of anatase TiO2 particles with UV enhances their cytotoxic and genotoxic potential in human hepatoma HepG2 cells. Journal of Hazardous Materials, 196, 145–152.
Piccinno F., Gottschalk F., Seeger S., Nowack B. (2012). Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. Journal of Nanoparticle Research 14. doi:10.1007/s11051-012-1109-9
Schmidt, J., & Vogelsberger, W. (2006). Dissolution kinetics of titanium dioxide nanoparticles the observation of an unusual kinetic size effect. Journal of Physical Chemistry B, 110, 3955–3963. doi:10.1021/jp055361l.
Solecka, J., Ziemska, J., Rajnisz, A., Laskowska, A., & Guśpiel, A. (2013). Actinomycetes—occurrence and production of biologically active compounds. Postępy Mikrobiologii, 52, 83–91.
Stankovic, N., Radulovic, V., Petkovic, M., Vuckovic, I., Jadranin, M., Vasiljevic, B., & Nikodinovic-Runic, J. (2012). Streptomyces sp. JS520 produces exceptionally high quantities of undecylprodigiosin with antibacterial, antioxidative, and UV-protective properties. Applied Microbiology and Biotechnology, 96, 1217–1231. doi:10.1007/s00253-012-4237-3.
Tung, W. S., & Daoud, W. A. (2009). New approach toward nanosized ferrous ferric oxide and Fe3O4-doped titanium dioxide photocatalysts. ACS Applied Materials & Interfaces, 1, 2453–2461.
Yamada, H., Urata, C., Aoyama, Y., Osada, S., Yamauchi, Y., & Kuroda, K. (2012). Preparation of colloidal mesoporous silica nanoparticles with different diameters and their unique degradation behavior in static aqueous systems. Chemistry of Materials, 24, 1462–147. doi:10.1021/cm3001688.
Weir, A., Westerhoff, P., Fabricius, L., & Goetz, N. (2012). Titanium dioxide nanoparticles in food and personal care products. Environmental Science and Technology, 46, 2242–2250. doi:10.1021/es204168d.
Ziemniak, S. E., Jones, M. E., & Combs, K. E. S. (1993). Solubility behavior of titanium(IV) oxide in alkaline media at elevated temperatures. Journal of Solution Chemistry, 22, 601–623. doi:10.1007/BF00646781.
Acknowledgments
The authors are grateful for the financial support of the National Science Centre Poland within the PRELUDIUM Programme (2011/03/N/ST5/04696). We also thank Dr. Jonathan Winfield for his assistance in improvement of the text’s clarity and language.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 354 kb)
Rights and permissions
About this article
Cite this article
Augustyniak, A., Cendrowski, K., Nawrotek, P. et al. Investigating the Interaction Between Streptomyces sp. and Titania/Silica Nanospheres. Water Air Soil Pollut 227, 230 (2016). https://doi.org/10.1007/s11270-016-2922-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-016-2922-z