Abstract
Purpose
Intraoperative molecular imaging with tumor-targeting fluorophores offers real-time detection of neoplastic tissue. The second window indocyanine green (SWIG) technique relies on passive accumulation of indocyanine green (ICG), a near-infrared fluorophore, in neoplastic tissues. In this study, we explore the ability of SWIG to detect neoplastic tissue and to predict postoperative magnetic resonance imaging (MRI) findings intraoperatively.
Procedures
Retrospective data were collected from 36 patients with primary high-grade gliomas (HGG) enrolled as part of a larger trial between October 2014 and October 2018. Patients received systemic ICG infusions at 2.5–5 mg/kg 24 h preoperatively. Near-infrared fluorescence was recorded throughout the case and from biopsy specimens. The presence/location of residual SWIG signal after resection was compared to the presence/location of residual gadolinium enhancement on postoperative MRI. The extent of resection was not changed based on near-infrared imaging.
Results
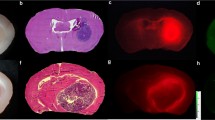
All 36 lesions demonstrated strong near-infrared fluorescence (signal-to-background = 6.8 ± 2.2) and 100 % of tumors reaching the cortex were visualized before durotomy. In 78 biopsy specimens, near-infrared imaging demonstrated higher sensitivity and accuracy than white light for diagnosing neoplastic tissue intraoperatively. Furthermore, near-infrared imaging predicted gadolinium enhancement on postoperative MRI with 91 % accuracy, with visualization of residual enhancement as small as 0.3 cm3. Patients with no residual near-infrared signal after resection were significantly more likely to have complete resection on postoperative MRI (p value < 0.0001).
Conclusions
Intraoperative imaging with SWIG demonstrates highly sensitive detection of HGG tissue in real time. Furthermore, post-resection near-infrared imaging correlates with postoperative MRI. Overall, our findings suggest that SWIG can provide surgeons with MRI-like results in real time, potentially increasing resection rates.





Similar content being viewed by others
References
Lacroix M, Abi-Said D, Fourney DR et al (2001) A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg 95:190–198
Sanai N, Polley M-Y, McDermott MW et al (2011) An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg 115:3–8
Stummer W, Kamp MA (2009) The importance of surgical resection in malignant glioma. Curr Opin Neurol 22:645–649
Sanai N, Berger MS (2011) Extent of resection influences outcomes for patients with gliomas. Rev Neurol 167:648–654
Sanai N, Berger MS (2008) Glioma extent of resection and its impact on patient outcome. Neurosurgery 62:753–764
Berkmann S, Schlaffer S, Nimsky C et al (2014) Intraoperative high-field MRI for transsphenoidal reoperations of nonfunctioning pituitary adenoma. J Neurosurg
Kuo JS, Barkhoudarian G, Farrell CJ et al (2016) Congress of neurological surgeons systematic review and evidence-based guideline on surgical techniques and technologies for the management of patients with nonfunctioning pituitary adenomas. Neurosurgery 79:E536–E538
Sheehan J, Lee C-C, Bodach M et al (2016) Congress of neurological surgeons systematic review and evidence-based guideline for the management of patients with residual or recurrent nonfunctioning pituitary adenomas. Neurosugery 79:539–540
Rainer Wirtz WS, Albert FK, Schwaderer M et al (2016) The benefit of neuronavigation for neurosurgery analyzed by its impact on glioblastoma surgery. Neurol Res
Sherman JH, Hoes K, Marcus J et al (2011) Neurosurgery for brain tumors: update on recent technical advances. Curr Neurol Neurosci Rep 11:313–319. https://doi.org/10.1007/s11910-011-0188-9
Mislow JMK, Golby AJ, Black PM (2009) Origins of intraoperative MRI. Neurosurg Clin N Am 20:137–146
Li P, Qian R, Niu C, Fu X (2017) Impact of intraoperative MRI-guided resection on resection and survival in patient with gliomas: a meta-analysis. Curr Med Res Opin 33:621–630
Napolitano M, Vaz G, Lawson TM et al (2014) Glioblastoma surgery with and without intraoperative MRI at 3.0T. Neurochirurgie 60:143–150
Kubben PL, Scholtes F, Schijns OEMG et al (2014) Intraoperative magnetic resonance imaging versus standard neuronavigation for the neurosurgical treatment of glioblastoma: a randomized controlled trial. Surg Neurol Int 5:70
Hatiboglu MA, Weinberg JS, Suki D et al (2009) Impact of intraoperative high-field magnetic resonance imaging guidance on glioma surgery. Neurosurgery 64:1073–1081
Senft C, Bink A, Franz K et al (2011) Intraoperative MRI guidance and extent of resection in glioma surgery: a randomised, controlled trial. Lancet Oncol. https://doi.org/10.1016/S1470-2045(11)70196-6
Kubben PL, ter Meulen KJ, Schijns OEMG et al (2011) Intraoperative MRI-guided resection of glioblastoma multiforme: a systematic review. Lancet Oncol 12:1062–1070
Stummer W, Stocker S, Wagner S et al (1998) Intraoperative detection of malignant gliomas by 5-aminolevulinic acid-induced porphyrin fluorescence. Neurosurgery 42:518–525 discussion 525-6
Stummer W, Pichlmeier U, Meinel T, et al (2006) Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. 7:392–401
van Dam GM, Themelis G, Crane LMA et al (2011) Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: first in-human results. Nat Med 17:1315–1319
Elliott JT, Marra K, Evans LT et al (2017) Simultaneous in vivo fluorescent markers for perfusion, protoporphyrin metabolism and EGFR expression for optically guided identification of orthotopic glioma HHS Public Access. Clin Cancer Res 23:2203–2212
Stummer W, Suero Molina E (2017) Fluorescence Imaging/agents in tumor resection. Neurosurg Clin N Am 28:569–583
Frangioni JV (2003) In vivo near-infrared fluorescence imaging. Curr Opin Chem Biol 7:626–634
Padalkar MV, Pleshko N (2015) Wavelength-dependent penetration depth of near infrared radiation into cartilage. Analyst 140:2093–2100
Lee JYK, Pierce JT, Thawani JP, et al. (2017) Near-infrared fluorescent image-guided surgery for intracranial meningioma. J Neurosurg 1–11.
Lee JYK, Pierce JT, Zeh R et al (2017) Intraoperative near-infrared optical contrast can localize brain metastases. World Neurosurg 106:120–130
Lee JYK, Thawani JP, Pierce J et al (2016) Intraoperative near-infrared optical imaging can localize gadolinium-enhancing gliomas during surgery. Neurosurgery 79:856–871
Cho SS, Salinas R, Lee JYK (2019) Indocyanine-green for fluorescence-guided surgery of brain tumors: evidence, techniques, and practical experience. Front Surg 6:11
Newton AD, Predina JD, Corbett CJ et al (2019) Optimization of second window indocyanine green for intraoperative near-infrared imaging of thoracic malignancy. J Am Coll Surg 228:188–197
Dsouza AV, Lin H, Henderson ER et al (2016) Review of fluorescence guided surgery systems: identification of key performance capabilities beyond indocyanine green imaging. J Biomed Opt:1
Cho SS, Zeh R, Pierce JT et al (2018) Comparison of near-infrared imaging camera systems for intracranial tumor detection. Mol Imaging Biol 20:213–220
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Vogelbaum MA, Jost S, Aghi MK et al (2012) Application of novel response/progression measures for surgically delivered therapies for gliomas: Response Assessment in Neuro-Oncology (RANO) Working Group. Neurosurgery 70:234–243 discussion 243-4
Ellingson BM, Wen PY, Cloughesy TF (2017) Modified criteria for radiographic response assessment in glioblastoma clinical trials. Neurotherapeutics 14:307–320
Coburger J, Wirtz CR (2019) Fluorescence guided surgery by 5-ALA and intraoperative MRI in high grade glioma: a systematic review. J Neuro-Oncol 141:533–546
Roder C, Bisdas S, Ebner FH et al (2014) Maximizing the extent of resection and survival benefit of patients in glioblastoma surgery: high-field iMRI versus conventional and 5-ALA-assisted surgery. Eur J Surg Oncol 40:297–304
Dg B, Bryant A, Vale L et al (2018) Intraoperative imaging technology to maximise extent of resection for glioma ( Review ). Cochrane Database of Systematic Reviews. https://doi.org/10.1002/14651858.CD012788.pub2
Jiang JX, Keating JJ, De Jesus EM et al (2015) Optimization of the enhanced permeability and retention effect for near-infrared imaging of solid tumors with indocyanine green. Am J Nucl Med Mol Imaging 5:390–400
Madajewski B, Judy BF, Mouchli A et al (2012) Intraoperative near-infrared imaging of surgical wounds after tumor resections can detect residual disease. Clin Cancer Res 18:5741–5751
Torchilin V (2011) Tumor delivery of macromolecular drugs based on the EPR effect. Adv Drug Deliv Rev 63:131–135
Weinmann H, Brasch R, Press W, Wesbey G (1984) Characteristics of gadolinium-DTPA complex: a potential NMR contrast agent. Am J Roentgenol 142:619–624
Onda N, Kimura M, Yoshida T, Shibutani M (2016) Preferential tumor cellular uptake and retention of indocyanine green for in vivo tumor imaging. Int J Cancer 139:673–682
Zeh R, Sheikh S, Xia L et al (2017) The second window ICG technique demonstrates a broad plateau period for near infrared fluorescence tumor contrast in glioblastoma. PLoS One 12:e0182034
Aime S, Caravan P (2009) Biodistribution of gadolinium-based contrast agents, including gadolinium deposition. J Magn Reson Imaging 30:1259–1267
Teraphongphom N, Kong CS, Warram JM, Rosenthal EL (2017) Specimen mapping in head and neck cancer using fluorescence imaging. Laryngoscope Investig Otolaryngol 2:447–452
Funding
Supported in part by the National Institutes of Health R01 CA193556 (SS), and the Institute for Translational Medicine and Therapeutics of the Perelman School of Medicine at the University of Pennsylvania (JYKL). Research reported in this publication was also supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000003 (JKYL). In addition, research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number TL1TR001880 (SSC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cho, S.S., Salinas, R., De Ravin, E. et al. Near-Infrared Imaging with Second-Window Indocyanine Green in Newly Diagnosed High-Grade Gliomas Predicts Gadolinium Enhancement on Postoperative Magnetic Resonance Imaging. Mol Imaging Biol 22, 1427–1437 (2020). https://doi.org/10.1007/s11307-019-01455-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-019-01455-x