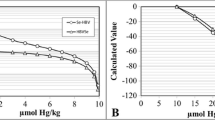
Abstract
Eating fish is often recommended as part of a healthful diet. However, fish, particularly large predatory fish, can contain significant levels of the highly toxic methylmercury (MeHg). Ocean fish in general also contain high levels of selenium (Se), which is reported to confer protection against toxicity of various metals including mercury (Hg). Se and Hg have a high mutual binding affinity, and each can reduce the toxicity of the other. This is an evolving area of extensive research and controversy with variable results in the animal and epidemiologic literature. MeHg is toxic to many organ systems through high affinity for –SH (thiol) ligands on enzymes and microtubules. Hg toxicity also causes oxidative damage particularly to neurons in the brain. Hg is a potent and apparently irreversible inhibitor of the selenoenzymes, glutathione peroxidases (GPX), and thioredoxin reductases (TXNRD) that are important antioxidants, each with a selenocysteine (SeCys) at the active site. Hg binding to the SeCys inhibits these enzymes, accounting in part for the oxidative damage that is an important manifestation of Hg toxicity, particularly if there is not a pool of excess Se to synthesize new enzymes. A molar excess of Se reflected in an Se:Hg molar ratio > 1 is often invoked as evidence that the Hg content can be discounted. Some recent papers now suggest that if the Se:Hg molar ratio exceeds 1:1, the fish is safe and the mercury concentration can be ignored. Such papers suggested that the molar ratio rather than the Hg concentration should be emphasized in fish advisories. This paper examines some of the limitations of current understanding of the Se:Hg molar ratio in guiding fish consumption advice; Se is certainly an important part of the Hg toxicity story, but it is not the whole story. We examine how Hg toxicity relates also to thiol binding. We suggest that a 1:1 molar ratio cannot be relied on because not all of the Se in fish or in the fish eater is available to interact with Hg. Moreover, in some fish, Se levels are sufficiently high to warrant concern about Se toxicity.

Similar content being viewed by others
Data availability
Data sharing is not applicable to this paper because no data sets were generated or analyzed during the study. All references used are listed in the reference section. All are available through PUBMED or at the URLs provided (updated 10 December 2020).
References
Abe T, Haga T, Kurokawa M (1975) Blockage of axoplasmic transport and depolymerisation of reassembled microtubules by methyl mercury. Brain Res 86:504–508
Ajsuvakova OP, Tinkov AA, Aschner M, Rocha JBT, Michalke B, Skalnaya MG, Skalny AV, Butnarin M, Dadar M, Sarac I, Aaseth J, Bjorklund G (2020) Sulfhydryl groups as targets of mercury toxicity. Coord Chem Rev 417:213343
Antunes Dos Santos A, Appel Hort M, Culbreth M, López-Granero C, Farina M, Rocha JB, Aschner M (2016) Methylmercury and brain development: a review of recent literature. J Trace Elem Med Biol 38:99–107
ATSDR (2003) Toxicological profile for selenium. Agency for Toxic Substances and Disease Research. Centers for Disease Control and Prevention, Atlanta https://www.atsdr.cdc.gov/toxprofiles/tp92.pdf [accessed 10 December 2020]
Azad AM, Frantzen S, Bank MS, Nilsen BM, Duinker A, Madsen L, Maage A (2019) Effects of geography and species variation on selenium and mercury molar ratios in Northeast Atlantic marine fish communities. Sci Total Environ 652:1482–1496
Baldissera MD, Souza CF, de Silva AS, Henn AS, Flores EMM, Baldisserotto B (2020) Diphenyl diselenide dietary supplementation alleviates behavior impairment and brain damage in grass carp (Ctenopharyngodon idella) exposed to methylmercury chloride. Comp Biochem Physiol C229:108674
Barbosa NBV, Rocha JBT, Zenia G, Emanuelli T, Beque MC, Braga AL (1998) Effect of organic forms of selenium on δ-aminolevulinate dehydratase from liver, kidney, and brain of adult rats. Toxicol Appl Pharmacol 149:243–253
Barone G, Storelli A, Mallamaci R, Storelli MM (2017) Comparative study on trace metal accumulation in liver of Mediterranean deep-sea fish and their selenium/mercury molar ratios. Water Air Soil Pollut 228:211
Beijer K, Jernelöv A (1978) Ecological aspects of mercury-selenium interactions in the marine environment. Environ Health Perspect 23:43–45
Benhar M (2018) Roles of mammalian glutathione peroxidase and thioredoxin reductase enzymes in the cellular response to nitrosative stress. Free Radic Biol Med 127:160–164
Bernstein AS, Oken E, de Ferranti S (2019) Council on Environmental Health; Committee on Nutrition. Fish, Shellfish, and Children’s Health: an assessment of benefits, risks, and sustainability. Pediatrics. 143(6):e20190999
Bloom N (1992) On the chemical form of mercury in edible fish and marine invertebrate tissue. Can J Fish Aquat Sci 49:1010–1017
Bonacker D, Stoiber T, Wang M, Böhm KJ, Prots I, Unger E, Thier R, Bolt HM, Degen GH (2004) Genotoxicity of inorganic mercury salts based on disturbed microtubule function. Arch Toxicol 78:575–583
Branco V, Canario J, Lu J, Holmgren A, Carvalho C (2012a) Mercury and selenium interaction in vivo: effects on thioredoxin reductase and glutathione peroxidase. Free Radic Biol Med 52:781–793
Branco V, Ramos P, Canario J, Lu J, Holmgren A, Carvalho C (2012b) Biomarkers of adverse response to mercury: histopathology versus thioredoxin reductase activity. J Biomed Biotechnol Article 3598879:1–9
Brandão R, Moresco RN, Bellé LP, Leite MR, de Freitas ML, Bianchini A, Nogueira CW (2011) Diphenyl diselenide potentiates nephrotoxicity induced by mercuric chloride in mice. J Appl Toxicol 31(8):773–782
Bridges CC, Zalups RK (2010) Transport of inorganic mercury and methylmercury in target tissues and organs. J Toxicol Environ Health B Crit Rev 13(5):385–410
Budtz-Jorgensen E, Grandjean P, Weihe P (2007) Separation of risks and benefits of seafood intake. Environ Health Perspect 115:323–327
Burger J (2009) Risk to consumers from mercury in bluefish (Pomatomus saltatrix) from New Jersey: size, season and geographical effects. Environ Res 1099:803–811
Burger J (2012) Selenium: mercury molar ratios in fish from the Savannah River: implications for risk management. J. Risk Res 15:627–644
Burger J, Gochfeld M (2011) Mercury and selenium levels in 19 species of saltwater fish from New Jersey as a function of species, size and season. Science Total Envron 409:1418–1429
Burger J, Gochfeld M (2012) Selenium and mercury molar ratios in saltwater fish from New Jersey: individual and species variability complicate use in human health fish consumption advisories. Environ Res 114:12–23
Burger J, Gochfeld M (2013) Selenium/mercury molar ratios in freshwater, marine, and commercial fish from the USA: variation, risk and health management. Rev Environ Health 28:129–143
Burger J, Gochfeld M (2020) Importance of biomonitoring levels of selenium, mercury, and selenium:mercury molar ratios in selected species in Northeastern United States estuaries: risks to biota and humans. J Environ Sci Pollution Res, this issue
Burk RF, Hill KE (2015) Regulation of selenium metabolism and transport. Annu Rev Nutr 35:109–134
Cabañero AL, Carvalho C, Madrid Y, Batoreu C, Camara C (2005) Quantification and speciation of mercury and selenium in fish samples of high consumption in Spain and Portugal. Biol Trace Elem Res 103:17–35
Caito SW, Jackson BP, Punshon T, Scrimale T, Grier A, Gill SR, Love TM, Watson GE, van Wijngaarden E, Rand MD (2018) Variation in methylmercury metabolism and elimination status in humans following fish consumption. Toxicol Sci 161:443–453
Cappon CJ, Smith JC (1982) Chemical form and distribution of mercury and selenium in edible seafood. J Anal Toxicol 6:10–21
Carneiro MF, Grotto D, Barbosa F Jr (2014) Inorganic and methylmercury levels in plasma are differentially associated with age, gender, and oxidative stress markers in a population exposed to mercury through fish consumption. J Toxicol Environ Health A77:69–79
Carvalho CML, Chew E, Hashemy SI, Lu J, Holmgren A (2008) Inhibition of the human thioredoxin system. A molecular mechanism of mercury toxicity J Biol Chem 283:11913–11923
Carvalho CM, Lu J, Zhang X, Arnér ES, Holmgren A (2011) Effects of selenite and chelating agents on mammalian thioredoxin reductase inhibited by mercury: implications for treatment of mercury poisoning. FASEB J 25:370–381
Carvalho LVB, Hacon SS, Vega CM, Vieira JA, Larentis AL, Mattos RCO, Valente D, Costa-Amaral IC, Mourão SGP, Oliveira BFA (2019) Oxidative stress levels induced by mercury exposure in Amazon juvenile populations in Brazil. Int J Environ Res Public Health 16(15):2682
Chen L, Zhang J, Zhu Y, Zhang Y (2015) Molecular interaction of inorganic mercury(ii) with catalase: a spectroscopic study in combination with molecular docking† RSC Advances No.97. 2015 https://pubs.rsc.org/en/content/articlelanding/2015/ra/c5ra15301h#!divAbstract [accessed 10 December 2020]
Choi AL, Budtz-Jørgensen E, Jørgensen PJ, Steuerwald U, Debes F, Weihe P, Grandjean P (2008) Selenium as a potential protective factor against mercury developmental neurotoxicity. Environ Res 107:45–52
Clarkson TW (1972) The pharmacology of mercury compounds. Annu Rev Pharmacol 12:375–406
Cusack LK, Eagles-Smith C, Hardin AK, Kile M, Stone D (2017) Selenium:mercury molar ratios in freshwater fish in the Columbia River Basin: potential applications for specific fish consumption advisories. Biol Trace Elem Res 18:136–146
Cuvin-Aralar ML, Furness RW (1991) Mercury and selenium interaction: a review. Ecotoxicol Environ Saf 21:348–364
Dang F, Wang W-X (2011) Antagonistic interaction of mercury and selenium in a marine fish is dependent on their chemical species. Environ Sci Technol 45:3116–3122
Dauplais M, Lazard M, Blanquet S, Plateau P (2013) Neutralization by metal ions of the toxicity of sodium selenide. PLoS One 8(1):e54353
Domingo JL (2016) Nutrients and chemical pollutants in fish and shellfish. Balancing health benefits and risks of regular fish consumption. Crit Rev Food Sci Nutr 56:979–988
Donald DB (2016) Relationships for mercury and selenium in muscle and ova of gravid freshwater fish. Environ Monit Assess 188:582
Dyrssen D, Wedborg M (1991) The Sulphur-mercury (II) system in natural waters. Water Air Soil Pollut 56:507–519
Eigsti OJ, Dustin P Jr, Gay-Winn N (1949) On the discovery of the action of colchicine on mitosis in 1889. Science 110:692
El-Begearmi MM, Sunde ML, Ganther H (1977) A mutual protective effect of mercury and selenium in Japanese quail. Poult Sci 56:313–322
Elhodaky M, Diamond AM (2018) Selenium-binding protein 1 in human health and disease. Int J Mol Sci 19(11):3437
EPA (2011) National listing of fish advisories. U.S. Environmental Protection Agency. https://19january2017snapshot.epa.gov/sites/production/files/2015-06/documents/technical-factsheet-2011.pdf [accessed 10 December 2020]
EPA (2020) Choose fish and shellfish wisely: fish and shellfish advisories and safe eating guidelines. U.S. Environmental Protection Agency http://www.epa.gov/choose-fish-and-shellfish-wisely/fish-and-shellfish-advisories-and-safe-eating-guidelines#guidelines [accessed 10 December 2020]
Farina M, Aschner M (2019) Glutathione antioxidant system and methylmercury-induced neurotoxicity: an intriguing interplay. Biochim Biophys Acta, Gen Subj 1863(12):129285
Farina M, Brandão R, Lara FS, Soares FA, Souza DO, Rocha JB (2003) Mechanisms of the inhibitory effects of selenium and mercury on the activity of delta-aminolevulinate dehydratase from mouse liver, kidney and brain. Toxicol Lett 139:55–66
Feng X et al (2007) Human exposure to methylmercury through rice intake in mercury mining areas, Guizhou Province, China. Environ Sci Technol 42:326–332
Frasco MF, Colletier JP, Weik M, Carvalho F, Guilbermino L, Stojan J, Fournier D (2007) Mechanisms of cholinesterase inhibition by inorganic mercury. FEBS J 274:1849–1861
Fuhr BJ, Rabenstein DL (1973) Nuclear magnetic resonance studies of the solution chemistry of metal complexes. IX. The binding of cadmium, zinc, lead, and mercury by glutathione. J Am Chem Soc 95(21):6944–6950
Gad SC (2014) Methylmercury, pp 318-320 Wexler P (ed) Encyclopedia of Toxicology Academic Press, New York, 3rd edition
Ganther HE, Sunde ML (1974) Effect of tuna fish and selenium on the toxicity of methylmercury: a progress report. J Food Sci 39:1–5
Ganther HE, Goudie C, Sunde ML, Kopecky MJ, Wagner P, Oh S-H, Hoekstra WG (1972) Selenium relation to decreased toxicity of methylmercury added to diets containing tuna. Science 175:1122–1124
García-Barrera T, Moro GR, Acosta SR, Borrego AA, Leblic BC, Abril N, Roldan FN, Gómez-Ariz JL (2019) Metabolic impairments caused by “chemical cocktails” in mammals and the protective role of selenium. Keynote Lecture, 3rd International Caparica Conference on Pollutant Toxic Ions & Molecules, Caparica, Portugal
Gardner MK, Zanic M, Howard J (2013) Microtubule catastrophe and rescue. Curr Opin Cell Biol 25:14–22
Gerson JR, Walters DM, Eagles-Smith CA, Bernhardt ES, Brandt JE (2020) Do two wrongs make a right? Persistent uncertainties tegarding environmental selenium–mercury interactions. Environ Sci Technol 54:9228–9234
Gochfeld M (2003) Cases of mercury exposure, bioavailability, and absorption. Ecotoxicol Environ Saf 56:174–179
Gochfeld M, Burger J (2005) Good fish/bad fish: a composite benefit-risk by dose curve. NeuroToxicology 26:511–520
Gochfeld M, Burger J, Jeitner C, Donio M, Pittfield T (2012) Seasonal, locational and size variations in mercury and selenium levels in striped bass (Morone saxatilis) from New Jersey. Environ Res 112:8–19
Goshima G, Vale RD (2003) The roles of microtubule-based motor proteins in mitosis comprehensive RNAi analysis in the Drosophila S2 cell line. J Cell Biol 162:1003–1016
Graff RD, Reuhl KR (1996) Chapter 38: Cytoskeletal toxicity of heavy metals, pp 639–658 in toxicology of metals (Chang LW, ed) CRC-Lewis, Boca Raton FL
Grandjean P, Satoh H, Murata K, Eto K (2010) Adverse effects of methylmercury: environmental health research implications. Environ Health Perspect 118:1137–1145
Hachiya N (2006) The history and the present of Minamata---Entering the second half century. JMAJ 49:112–118
Hamilton SJ (2004) Review of selenium toxicity in the aquatic food chain. Sci Total Environ 326:1–31
Heinz GH, Hoffman DJ, Klimstra JD, Stebbins KR (2012) A comparison of the teratogenicity of methylmercury and selenomethionine injected into bird eggs. Arch Environ Contam Toxicol 62:519–528
Hill CH (1975) Interrelationships of selenium with other trace elements. Fed Proc 14:2096–2100
Hoffman DJ (2002) Role of selenium toxicity and oxidative stress in aquatic birds. Aquat Toxicol 57:11–26
Hoffman DJ, Heinz GH (1998) Effects of mercury and selenium on glutathione metabolism and oxidative stress in mallard ducks. Environ Toxicol Chem 17:161–165
Hunter D (1978) Diseases of occupations, 6th edn. Hodder and Stoughton, London
Hurna E, Siklenka P, Hurna S (1997) Effect of selenium on cadmium genotoxicity investigated by micronucleus assay. Vet Med (Praha) 42:339–342
IARC (1993) Beryllium, cadmium, mercury and exposures in the glass manufacturing industry. IARC monographs on the evaluation of carcinogenic risks to humans, vol 58. International Agency for Research on Cancer, Lyon https://www.ncbi.nlm.nih.gov/books/NBK499756/ [Accessed 10 December 2020]
Imura N, Miura K, Inokawa M, Nakada S (1980) Mechanism of methylmercury cytotoxicity: by biochemical and morphological experiments using cultured cells. Toxicology 17:241–254
IOM (2006) Dietary reference intakes: Institute of Medicine, National Academies Press, The Essential Guide to Nutrient Requirements Washington DC pp 313–19, 415–22 https://doi.org/10.17226/11537. [Accessed 12 December 2020]
Jagadeesan G, Sankarsami Pillai S (2007) Hepatoprotective effects of taurine against mercury induced toxicity in rats. J Environ Biol 28:753–756
Jenko K, Karouna-Reier NK, Hoffman DJ (2012) Gene expression, glutathione status, and indicators of hepatic oxidative stress in laughing gull (Larus atricilla) hatchlings exposed to methylmercury. Environ Toxicol Chem 31:2588–2596
Jensen S, Jernelöv A (1969) Biological methylation of mercury in aquatic organisms. Nature 223(5207):753–754
Johnson RC, Stewart AF, Limburg KE, Huang R, Cocherell D, Feyrer F (2020) Lifetime chronicles of selenium exposure linked to deformities in an imperiled migratory fish. Environ Sci Technol 54:2892–2901
Kade IJ (2012) Mercury toxicity on sodium pump and organoseleniums intervention: a paradox. J Biomed Biotechnol 2012:924549
Kaneko JJ, Ralston NVC (2007) Selenium and mercury in pelagic fish in the central North Pacific near Hawaii. Biol Trace Elem Res 119:242–254
Kasprzak K (1997) Oxidative DNA damage in metal-induced carcinogenesis. In: Chang LW (ed) Toxicology of Metals. CRC Lewis Publ, Boca Raton, pp 299–320
Komsta-Szumska E, Reuhl KE, Miller DR (1983) Effect of selenium on distribution, demethylation and excretion of methylmercury by the guinea pig. J Toxicol Environ Health 12:775–785
Korbas M, O’Donoghue JL, Watson GE, Pickering IJ, Singh SP, Myers GJ, Clarkson TW, George GN (2010) The chemical nature of mercury in human brain following poisoning or environmental exposure. ACS Chem Neurosci 1:810–818
Kurland LT, Faro SN, Siedler H (1960) Minamata Disease. The outbreak of a neurologic disorder in Minamata, Japan, and its relationship to the ingestion of seafood contaminated by mercuric compounds. World Neurol 1960:370–395
Lemire M, Fillion M, Frenette B, Passos CJ, Guimarães JR, Barbosa F Jr, Mergler D (2011) Selenium from dietary sources and motor functions in the Brazilian Amazon. Neurotoxicology. 32:944–953
Liu Y, Zhang W, Zhao J, Lin X, Liu J, Cui L, Gao U, Zhang TL, Li B, Li YF (2018) Selenoprotein P as the major transporter for mercury in serum from methylmercury-poisoned rats. J Trace Elem Med Biol 50:589–595
Liu Y, Ji J, Zhang W, Suo Y, Zhao J, Lin X, Cui L, Bai L, Hu H, Chen C, Li Y (2019) Selenium modulated gut flora and promoted decomposition of methylmercury in methylmercury-poisoned rats. Ecotoxicol Environ Saf 185:109720
Lubos E, Loscalzo J, Handy DE (2011) Glutathione peroxidase-1 in health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal 15:1957–1997
Magos L, Webb M (1980) The interactions of selenium with cadmium and mercury. Crit Rev Toxicol 8(1):1–42
McAlpine D, Araki S (1958) Minamata disease: an unusual neurological disorder caused by contaminated fish. Lancet 2(7047):629–631
Miller DM, Woods JS (1993) Urinary porphyrins as biological indicators of oxidative stress in the kidney. Interaction of mercury and cephaloridine. Biochem Pharmacol 46:2235–2241
Mulder PJ, Lie E, Eggen GS, Ciesielski TM, Berg T, Skaare JU, Jenssen BM, Sørmo EG (2012) Mercury in molar excess of selenium interferes with thyroid hormone function in free-ranging freshwater fish. Environ Sci Technol 46:9027–9037
Mykkanen HM, Wasserman RH (1990) Relationship of membrane-bound sulfhydryl groups to vitamin D-stimulated uptake of [75Se]selenite by the brush border membrane vesicles from chick duodenum. J Nutr 120:882–888
Nesci S, Trombetti F, Pirini M, Ventrella V, Pagliarani A (2016) Mercury and protein thiols: stimulation of mitochondrial F1Fo-ATPase and inhibition of respiration. Chemico-Biol Interact 260:42–49
New Jersey Mercury Task Force (2001) Final report Vol 1 Executive Summary & Recommendations. New Jersey Department of Environmental Protection, Trenton, https://www.state.nj.us/dep/dsr/nj-mercury-volume1.PDF [accessed 10 December 2020]
Palacios Ò, Capdevila M (2013) Metallothioneins and mercury. In: Kretsinger RH, Uversky VN, Permyakov EA (eds) Encyclopedia of Metalloproteins. Springer, New York. https://doi.org/10.1007/978-1-4614-1533-6_309 [accessed 10 December 2020]
Papp LV, Lu J, Holmgren A, Khanna KK (2007) From selenium to selenoproteins: synthesis, identity, and their role in human health. Antioxid Redox Signal 9:775–806
Parízek J, Ostádalová I (1967) The protective effect of small amounts of selenite in sublimate intoxication. Experientia 23:142–143
Parízek J, Ostádalová I, Kalouskova J Babicky A, Benes J (1971) The detoxifying effects of selenium, interrelations between compounds of selenium and certain metals, pp. 85-122 In: Metz W, Cornatzer WE (eds) Newer Trace Elements in Nutrition Marcel Dekker, New York
Penglase S, Hamre K, Ellinngsen (2014) Selenium and mercury have a synergistic negative effect on fish reproduction. Aquat Toxicol 149(16–24):2014
Picaud T, Desbois A (2006) Interaction of glutathione reductase with heavy metal: the binding of Hg(II) or Cd(II) to the reduced enzyme affects both the redox dithiol pair and the flavin. Biochemistry 45:15829–15837
Polak-Juszczak L (2015) Selenium and mercury molar ratios in commercial fish from the Baltic Sea: additional risk assessment criterion for mercury exposure. Food Control 50:881–888
Polevoy C, Arbuckle TE, Oulhote Y, Lanphear BP, Cockell KA, Muckle G, Saint-Amour D (2020) Prenatal exposure to legacy contaminants and visual acuity in Canadian infants: a maternal-infant research on environmental chemicals study (MIREC-ID). Environ Health 19(1):14
Poopal RK, Ramesh M, Dinesh B (2013) Short-term mercury exposure on Na+/K+-ATPase activity and ionoregulation in gill and brain of an Indian major carp, Cirrhinus mrigala. J Trace Elem Med Biol 27:70–75
Ralston NVC, Raymond LJ (2018) Mercury’s neurotoxicity is characterized by its disruption of selenium biochemistry. Biochim Biophys Acta Gen Subj 1862:2405–2416
Ralston NV, Blackwell JL 3rd, Raymond LJ (2007) Importance of molar ratios in selenium-dependent protection against methylmercury toxicity. Biol Trace Elem Res 119:255–268
Ralston NV, Ralston CR, Blackwell JL 3rd, Raymond LJ (2008) Dietary and tissue selenium in relation to methylmercury toxicity. Neurotoxicology 29:802–811
Ralston NVC, Ralston CR, Raymond LJ (2016) Selenium health benefit values: updated criteria for mercury risk assessments. Biol Trace Elem Res 171:262–269
Ralston NVC, Kaneko JJ, Raymond LJ (2019) Selenium health benefit values provide a reliable index of seafood benefits vs. risks. J Trace Elem Med Biol 55:50–57
Ramel C (1969) Genetic effects of organic mercury compounds. I. Cytological investigations on Allium roots. Hereditas 61:208–230
Ramel C, Magnusson J (1969) Genetic effects of organic mercury compounds. II Chromosome segregation in Drosophila melanogaster. Hereditas 61:231–254
Rand MD, Caito SW (2019) Variation in the biological half-life of methylmercury in humans: methods, measurements and meaning. Biochim Biophysica Acta Gen Sub 1863(12):129301
Rayman MP (2012) Selenium and human health. Lancet 379:1256–1268
Raymond LJ, Ralston NVC (2004) Mercury:selenium interactions and health implications. Seychelles Med Dent J(Special Issue) 7:72–77
Reuhl KR (1988) Role of cytoskeletal damage in congenital methylmercury poisoning. In: Singer TP, Castanoli N, Wang CC (eds) Molecular basis of the action of drugs and toxic substances. Walter de Gruyter, New York, pp 211–224
Reyes-Avila AD, Laws ED, Herrman AD, DeLaune RD, Blanchard TP (2019) Mercury and selenium levels, and Se:Hg molar ratios in freshwater fish from South Louisiana. J Environ Sci Health (Part A) 54:238–245
Rimm EB, Appel LJ, Chiuve SE, Diousse L, Engler MB, Kris-Eterton PM, Mazaffarian D, Siscovick DS, Lichtenstein AH (2018) Seafood long-chain n-3 polyunsaturated fatty acids and cardiovascular disease: a science advisory from the American heart association. Circulation 138:e35–e47. https://doi.org/10.1161/CIR.0000000000000574
Rocha JB, Pereira ME, Emanuelli T, Christofari RS, Souza DO (1995) Effect of treatment with mercury chloride and lead acetate during the second stage of rapid postnatal brain growth on delta-aminolevulinic acid dehydratase (ALA-D) activity in brain, liver, kidney and blood of suckling rats. Toxicology 100:27–37
Roman HA, Walsh TL, Couli BA, Dewailly E, Guallar E, Hattis D, Marian K, Schwartz J, Stern AH, Virtanen JK, Rice G (2012) Evaluation of the cardiovascular effects of methylmercury exposures: current evidence supports development of a dose-response function for regulatory benefits analysis. Environ Health Perspect 119:607–614
Rubino FM (2015) Toxicity of glutathione-binding metals: a review of targets and mechanisms. Toxics 26:20–62
Sager PR, Doherty RA, Olmsted JB (1983) Interaction of methylmercury with microtubules in cultured cells and in vitro. Exp Cell Res 146:127–137
Santos AB, Silva WL (2017) New evaluation of selenium mercury ratios in fish and crabs from an impacted tropical estuary in southeastern Brazil. Int J Environ Sci Natural Resources 2(3):555589
Schartup AT, Thackray CP, Qureshi A, Dassuncao C, Gillespie K, Hanke A, Sunderland EM (2019) Climate change and overfishing increase neurotoxicant in marine predators. Nature 572:648–650
Shreenath AP, Ameer MA, Dooley J (2020). Selenium deficiency. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, https://pubmed.ncbi.nlm.nih.gov/29489289/ [accessed 10 December 2020]
Sies H (2015) Oxidative stress: a concept in redox biology and medicine. Redox Biol 4:180–183
Skerfving S (1978) Interaction between selenium and methylmercury. Environ Health Perspect 25:57–65
Sørmo EG, Ciesielski TM, Øverjordet IB, Lierhagen S, Eggen GS, Berg T, Jenssen BM (2011) Selenium moderates mercury toxicity in free-ranging freshwater fish. Environ Sci Technol 45:6561–6566
Spallholz JE (1993) On the nature of selenium toxicity and carcinostatic activity. Free Radic Biol Med 17:45–64
Spiller HA (2018) Rethinking mercury: the role of selenium in the pathophysiology of mercury toxicity. Clin Toxicol 56:313–326
Spulber S, Raciti M, Dulko-Smith B, Lupu D, Ruegg J, Nam K, Ceccatelli S (2018) Methylmercury interferes with glucocorticoid receptor: potential role in the mediation of developmental neurotoxicity. Toxicol Appl Pharmacol 354:94–100
Squadrone S, Benedetto A, Brizio P, Prearo M, Abete MC (2015) Mercury and selenium in European catfish (Silurus glanis) from northern Italian Rivers: can molar ratio be a predictive factor for mercury toxicity in a top predator? Chemosphere 119:24–30
Stoiber T, Bonacker D, Böhm KJ, Bolt HM, Thier R, Degen GH, Unger E (2004) Disturbed microtubule function and induction of micronuclei by chelate complexes of mercury(II). Mutat Res 563:97–106
Stratton A, Ericksen M, Harris TV, Symmonds N, Silverstein TP (2017) Mercury(II) binds to both of chymotrypsin’s histidines, causing inhibition followed by irreversible denaturation/aggregation. Protein Sci 26:292–305
Sugiura Y, Tamai Y, Tanaka H (1978) Selenium protection against mercury toxicity: high binding affinity of methylmercury by selenium-containing ligands in comparison with sulfur-containing ligands. Bioinorg Chem 9:167–180
Surai PF (2006) Selenium in nutrition and health. Nottingham Univ Press, Nottingham, p 974
Temel Y, Taysi MS (2019) The effect of mercury chloride and boric acid on rat erythrocyte enzymes. Biol Trace Elem Res 191:177–182
Ulusoy S, Mol S, Karakula FS, Kahraman AE (2019) Selenium-mercury balance in commercial fish species in Turkish waters. Biol Trace Elem Res 191:207–213
Vahter ME, Mottet NK, Friberg LT, Lind SB, Charleston JS, Burbacher TM (1995) Demethylation of methyl mercury in different brain sites of Macaca fascicularis monkeys during long-term subclinical methyl mercury exposure. Toxicol Appl Pharmacol 143:273–284
Vega-Sánchez B, Ortega-García S, Ruelas-Inzunza J, Frías-Espericueta M, Escobar-Sánchez O, Jara-Marini M (2020) Selenium and mercury in Dolphinfish (Coryphaena hippurus) from the Gulf of California: inter-annual variations and selenium health benefit value. Environ Sci Pollut Res Int 27:2311–2318
Vinceti M, Wei ET, Malagoli C, Bergomi M, Vivoli G (2001) Adverse health effects of selenium in humans. Rev Environ Health 16:233–251
Vogel DG, Margolis RL, Mottet NK (1989) Analysis of methyl mercury binding sites on tubulin subunits and microtubules. Pharmacol Toxicol 64:196–201
Wang X, Horisberger JD (1996) Mercury binding site on Na+/K(+)-ATPase: a cysteine in the first transmembrane segment. Mol Pharmacol 50:687–691
Wang X, Wu L, Sun J, Wei Y, Zhou Y, Rao Z, Yuan L, Liu X (2018) Mercury concentrations and Se:Hg molar ratios in flyingfish (Exocoetus volitans) and squid (Uroteuthis chinensis). Bull Environ Contam Toxicol 101:42–48
Wasteneys GO, Cadrin M, Reuhl KR, Brown DL (1988) The effects of methylmercury on the cytoskeleton of murine embryonal carcinoma cells. Cell Biol Toxicol 4:41–60
Watanabe C (2002) Modification of mercury toxicity by selenium: practical importance? Tohoku J Exp Med 196:71–77
Weed R, Eber J, Rothstein A (1962) Interaction of mercury with human erythrocytes. J Gen Physiol 45:395–410
Woods JS, Ellis ME (1995) Up-regulation of glutathione synthesis in rat kidney by methyl mercury. Relationship to mercury-induced oxidative stress. Biochem Pharmacol 50:1719–1724
Yang D, Chen Y, Gunn JM, Belzile N (2008) Selenium and mercury in organisms: interactions and mechanisms. Environ Rev 16:71–92
Yarris L (1998) Mystery of vital cell protein solved after 30 years. Berkeley Lab Research News January 8:1998 https://www2.lbl.gov/Science-Articles/Archive/3D-tubulin.html [accessed 10 December 2020]
Zalups RK, Lash LH (1996) Interactions between glutathione and mercury in the kidney, liver, and blood. In: Chang LW (ed) Toxicology of Metals. CRC press-Lewis, Boca Raton, pp 145–163
Zalups RK, Lash LH (1997) Binding of mercury in renal brush-border and basolateral membrane-vesicles. Biochem Pharmacol 53:1889–1900
Zayas ZP, Ouerdane L, Mounicou S, Lobinski R, Monperrus M, Amouroux D (2014) Hemoglobin as a major binding protein for methylmercury in white-sided dolphin liver. Anal Bioanal Chem 406:1121–1129
Zhang Y, Roh YJ, Han S, Park I, Lee HM, Ok YS, Lee BC, Lee SR (2020) Role of selenoproteins in redox regulation of signaling and the antioxidant system: a review. Antioxidants 9(5):383
Acknowledgments
We thank our colleagues Michael Gallo, Jeffrey Laskin, Dan Morse, Ken Reuhl, and Helmut Zarbl who have shared their extensive knowledge of biochemistry and toxicology in general and mercury and selenium in particular. Nicholas Ralston provided valuable discussions, questioned our assumptions, and addressed our questions. We have benefitted from decades of discussions and collaborations on issues of fish consumption advice and advisories including Ned Groth, Philippe Grandjean, and Alan Stern. This paper benefitted greatly from two knowledgeable and thoughtful anonymous reviewers who encouraged us to re-examine and substantiate many of our assumptions. And thanks to our patients who came to our clinic with high mercury levels and symptoms of mercury poisoning from eating a lot of fish, usually for “health reasons.” Fortunately most of them have gotten better as their mercury levels declined. Our clinic colleagues Howard Kipen, Iris Udasin, Michael Pratt, Nancy Fiedler, and Rob Laumbach contributed with valuable discussions of differential diagnoses and management options. Christian Jeitner handled searches, manuscript preparation, formatting, and submission details in addition to preparing graphics for the oral presentations. Last but not least we thank Carlos Lodeiro Espiño and Jose Luis Capello for the invitation to participate in the 3rd PTIM conferences and present these ideas to an audience of diverse expertise.
Funding
This research was funded by the National Institute of Environmental Health Science Center for Environmental Exposure and Disease (NIH-NIEHS P30ES0050022), the USDA National Institute of Food and Agriculture (Hatch Multistate Project 1008906 through NJAES (Hatch NJ 12233, W4045), and the US DOE (DE-FC 01-06EW 07503 grant to the Consortium for Risk Evaluation with Stakeholder Participation (CRESP)). Travel support for J Burger was provided by the PTIM conference organization as a plenary speaker.
Author information
Authors and Affiliations
Contributions
Gochfeld and Burger both contributed to the conceptualization of the paper, literature reviews, and analysis of the concepts and data presented in the paper. All references are noted in the literature cited.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethical approval
The paper is a conceptualization and analysis of the published literature on the topic. No human subjects or animals were used in this paper.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
1. Fish have high nutritional value, but some also have high levels of methylmercury.
2. Mercury exerts toxic effects by binding to sulfhydryl (–SH) groups which are its most abundant ligand in the fish and in the human.
3. Mercury inhibits many enzymes by reacting with thiols and also selenols.
4. Mercury inhibits microtubule formation essential for cell structure, cellular transport, and cell division, by binding to –SH sites on the tubulin protein to prevent assembly.
5. Mercury has high affinity for selenium and causes oxidative damage by inhibiting selenoenzymes (e.g., GPX, TXNRD) that are major components of cells’ antioxidant defenses. Some believe that this is the major mechanism for Hg toxicity.
6. Exposure to methylmercury from fish poses a public health risk, although a great excess of Se may partially mitigate the risk by assuring an adequate supply of selenocysteine for selenoprotein replacement. However, the risk of Se toxicity should not be ignored.
7. The Se:Hg molar ratio provides information on the relative excess of Se, but people who eat fish frequently should avoid fish with high mercury content. Fish advisories should take into account the mercury concentration and not rely solely on the molar ratio.
Rights and permissions
About this article
Cite this article
Gochfeld, M., Burger, J. Mercury interactions with selenium and sulfur and the relevance of the Se:Hg molar ratio to fish consumption advice. Environ Sci Pollut Res 28, 18407–18420 (2021). https://doi.org/10.1007/s11356-021-12361-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-12361-7