Abstract
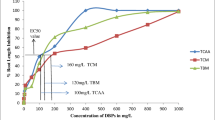
Chlorophenols are not only noticed in an effluvium of industries but also can emerge from the water treatment plants for domestic supply which poses a high threat for crop production and human health. Therefore, research on their risks to ecosystem and human health via ecotoxicological tests to derivate permissible environmental contaminant concentrations is necessary. The chlorophenols produced in the course of chlorination of potable water is an outcome of natural carboxylic acids/organic material and those chlorophenols occurred as emerging disinfection byproducts (EDBPs). Among chlorophenols, pentachlorophenol (PCP) has been recently identified as one of the important EDBPs. The main objective was to evaluate the PCP-induced genotoxicity and the oxidative damage in two plant species, i.e., Allium cepa and Vigna radiata. Genotoxicity of PCP was examined at three selected concentrations based on EC50 (half-maximal effective concentrations) values in both the plants along with the defense mechanism. EC50 value for A. cepa and V. radiata was 0.7 mg/L and 35 mg/L. Root length inhibition, DNA laddering, lipid peroxidation, H2O2 content, and antioxidant enzymatic assays evaluated revealed a dose-dependent response. PCP influenced defense enzyme glutathione peroxidase (GPX) and ascorbate peroxidase (APX) action in both plants and showed deprivement of catalase (CAT) with the increase of PCP concentrations. PCP-invaded toxicity management by these plants implied that A. cepa is more sensitive than V. radiata regarding PCP-induced toxicity.








Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Abu NE, Mba KC (2011) Mutagenicity testing of pharmaceutical effluents on Allium cepa root tip meristems. J Toxicol Environ Health Sci 3:44–51
Abusalama EA, Elhassan AM, Errami M, Salghi R (2014) Pesticides residues: endosulfan and DDT in Cow’s milk in Gezira state. Sudan Mor J Chem 2:125–135
Ali M, Sreekrishnan TR (2001) Aquatic toxicity from pulp and paper mill effluents: a review. Adv Environ Res 5(2):175–196
Araujo BS, Dec J, Bollag JM, Pletsch M (2006) Uptake and transformation of phenol and chlorophenols by hairy root cultures of Daucus carota, Ipomoea batatas, and Solanum aviculare. Chemosphere. 63(4):642–651
Ateeq M, Abul Farah M, Niamat A, Ahmad W (2002) Clastogenicity of pentachlorophenol, 2,4-D and butachlor evaluated by Allium root tip test. Mutat Res 514(1–2):105–113
ATSDR, (2001) Toxicological profile for pentachlorophenol. Agency for Toxic Substances and Disease Registry, Public Health Service, U.S. Department of Health and Human Services, Atlanta, GA, 316
Bakadir K, Kassale A, Barouni K, Lakhmiri R, Albourine A (2016) Retention of a compound of herbicides, 2,4-dichloro phenoxy acetic acid, to the soil in the absence and the presence of Cu(II) and Zn(II) cations. J Mater Environ Sci 7:1056–1063
Bashir F, Mahmooduzzafar Siddiqi TO, Iqbal M (2007) The antioxidative response system in Glycine max (L.) Merr. exposed to Deltamethrin, a synthetic pyrethroid insecticide. Environ Pollut 147:94–100
Behera M, Dhali D, Chityala S, Mandal T, Bhattacharya P, Dasgupta Mandal D (2015) Evaluation of the performance of Planococcus sp. TRC1 an indigenous bacterial isolate monoculture as bioremediator for tannery effluent. Desalin Water Treat 57(28):1–12
Behera M, Paul I, Paul SS, Mandal T, Mandal DDG (2019) Simultaneous o-cresol degradation and biosurfactant production by indigenous bacterial monoculture: kinetics and genotoxic risk assessment. Desalin Water Treat 144:116–128
Bull RJ, Krasner SW, Daniel PA, Bull RD (2001) Health effects and occurrence of disinfection by-products. Denver, CO., AWWAResearch Foundation ISBN 1-58321-0997
Chhabra R, Maronot R, Bucher J, Haseman J, Toft J, Hejtmancik M (1999) Toxicology and carcinogenesis studies of pentachlorophenol in rats. Toxicol Sci 48:14–20
Choudhury S, Panda SK (2004) Induction of oxidative stress and ultrastructural changes in moss Taxithelium Nepalese (Schwaegr.) Broth. under lead and arsenic phytotoxicity. Curr Sci 87:342–348
Collins AR (1999) Oxidative DNA damage, antioxidants and cancer. Bioessays. 3:228–246
Dixon DP, Lapthorn A, Edwards R (2002) Plant glutathione transferases. Genome Biol 3:1–10
Draper HH, Hadley M (1990) Malondialdehyde determination as an index of lipid peroxidation. Method Enzymol 86:421–431
Dunand C, Crèvecoeur M, Penel C (2007) Distribution of superoxide and hydrogen peroxide in Arabidopsis root and their influence on root development: possible interaction with peroxidases. New Phytol 174(2):332–341
Edwards R, Brazier-Hicks M, Dixon DP, Cummins I (2005) Chemical manipulation of antioxidant defenses in plants. Adv Bot Res 42:1–32
Farah M, Ateeq B, Ali M, Sabir R, Ahmad W (2004) Studies on lethal concentrations and toxicity stress of some xenobiotics on aquatic organisms. Chemosphere. 55:257–265
Farrington JA, Ebert M, Land EJ, Fletcher K, (2007) Bipyridylium quaternary salts and related compounds. V Pulse radiolysis studies of the reaction of paraquat radical with oxygen Implications for the mode of action of bipyridyl herbicides Biochim Biophys Acta 314: 372–381
Fatima RA, Ahmad M (2005) Certain antioxidant enzymes of Allium cepa as biomarkers for the detection of toxic heavy metals in wastewater. Sci Total Environ 346(1–3):256–273
Fiskesjö G (1985) The Allium test as a standard in environmental monitoring. Hereditas 102(1):99–112
Foy CD, Chaney RL, White MC (2003) The physiology of metal toxicity in plants. Annu Rev Plant Biol 29(1):511–566
Foyer CH, Halliwell B (1976) Presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta. 133:21–25
Fragiadakis A, Sotiriouand N, Korte F (1981) Absorption, balance and metabolism of 14C-2,4,6-trichlorophenol in hydro-ponic tomato plants. Chemosphere. 10:1315–1320
Fujita M, Fujita Y, Noutoshi Y, Takahashi F, Narusaka Y, YamaguchiShinozaki K (2006) Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Curr Opin Plant Biol 9:436–442
Gao JJ, Liu LH, Liu XR, Zhou HD, Huang SB, Wang ZJ (2008) Levels and spatial distribution of chlorophenols 2,4-dichlorophenol, 2,4,6- trichlorophenol, and pentachlorophenol in surface water of China. Chemosphere 71:1181–1187
Ge JC, Pan JL, Fel ZL, Wu GH, Giesy JP (2007) Concentrations of pentachlorophenol (PCP) in fish and shrimp in Jiangsu Province, China. Chemosphere 69:164–169
Ghosh M, Bandopadhyay M, Mukhopadhyay A (2010) Genotoxicity of titanium dioxide (TiO2) nanoparticles at two tropic levels: plant and human lymphocytes. Chemosphere 81:253–1262
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Góth L, Rass P, Páy A (2004) Catalase enzyme mutations and their association with diseases. Mol Diagn 8:141–149
Grant WF (1994) The present status of higher plant bioassays for the detection of environmental mutagens. Mutat Res 310(2):175–185
Haq I, Kumari V, Kumar S, Raj A, Lohani M, Bhargava RN (2016) Evaluation of the phytotoxic and genotoxic potential of pulp and paper mill effluent using Vigna radiata and Allium cepa. Adv Biol:10
Hartley-Whitaker J, Ainsworth G, Meharg AA (2001) Copper and arsenate induced oxidative stress in Holcus lanatus L. clones with differential sensitivity. Plant Cell Environ 24:13–22
Hattab S, Flores-Casseres S, Boussetta ML, Doumas H, Hernandez P, Banni LE (2016) Characterization of lead-induced stress molecular biomarkers in Medicago sativa plants. Environ Exp Bot 123:1–2
Hebert A, Forestier D, Lenes D, Benanou D, Jacob S, Arfi C, Lambolez L, Levi Y (2010) Innovative method for prioritizing emerging disinfection by-products (DBPs) in drinking water on the basis of their potential impact on public health. Water Res 44(10):3147–3165
Hechmi N, Ben Aissa N, Abdenaceur H, Jedidi N (2015) Uptake and bioaccumulation of pentachlorophenol by emergent wetland plant Phragmites australis (common reed) in cadmium co-contaminated soil. Int J Phytoremediation 17(1–6):109–116
Huang H, Ullah F, Zhou DZ, Yi M, Zhao Y (2019) Mechanisms of ROS regulation of plant development and stress responses. Front Plant Sci 10:800. https://doi.org/10.3389/fpls.2019.00800
Izabela J, Patryk O, Ewa S (2017) Toxicity of combined mixtures of nanoparticles to plants. J Hazard Mater 331:200–209
Kannan Upreti RK (2008) Influence of distillery effluent on germination and growth of mung bean (Vigna radiata) seeds. J Hazard Mater 153:609–615
Karuppanapandian T, Moon JC, Kim C, Manoharan K, Kim W (2011) Reactive oxygen species in plants: their generation, signal transduction, and scavenging mechanisms. AJCS. 5(6):709–725
Kono Y, Fridovich I (1982) Superoxide radicals inhibit catalase. J Biol Chem 257:5751–5754
Kucuk D, Liman R (2018) Cytogenetic and genotoxic effects of 2-chlorophenol on Allium cepa L. root meristem cells. Environ Sci Pollut Res 25:36117–36123
Kumari M, Anita M, Chandrasekaran N (2009) Genotoxicity of silver nanoparticles in Allium cepa. Sci Total Environ 407:5243–5246
Latorre A, Malmqvist A, Lacorte S, Welander T, Barceló D (2007) Evaluation of the treatment efficiencies of paper mill whitewaters in terms of organic composition and toxicity. Environ Pollut 147(3):648–655
Leme DM, Marin-Morales MA (2009) Allium cepa test in environmental monitoring: a review on its application. Mutat Res 682:71–81
Liman R, Ciğerci IH, Öztürk NS (2015) Determination of genotoxic effects of Imazethapyr herbicide in Allium cepa root cells by mitotic activity, chromosome aberration, and comet assay. Pestic Biochem Physiol 118:38–42
Liu Z, Hossain GS, Islas-Osuna MA, Mitchell DL, Mount DW (2000) Repair of UV damage in plants by nucleotide excision repair: Arabidopsis UVH1 DNA repair gene is a homolog of Saccharomyces cerevisiae Rad1. Plant J 21:519–528
Liu H, Weisman D, Ye YB, Cui B, Huang YH, Colon-Carmona A, Wang ZH (2009) An oxidative stress response to polycyclic aromatic hydrocarbon exposure is rapid and complex in Arabidopsis thaliana. Plant Sci 176:375–382
Maluszynska J, Juchimiuk J (2005) Plant genotoxicity: a molecular cytogenetic approach in plant bioassays. Arch Ind Hyg Toxicol 56(2):177–184
Martí E, Sierra J, Cáliz J, Montserrat G, Vila X, Garau MA, Cruañas R (2011) Ecotoxicity of chlorophenolic compounds depending on soil characteristics. Sci Total Environ 409:2707–2716
Martí E, Sierra J, Sánchez M, Cruañas R, Garau MA (2007) Ecotoxicological tests assessment of soils polluted by chromium (VI) or pentachlorophenol. Sci Total Environ 378:53–57
Mitton FM, Gonzalez M, Monserrat JM, Miglioranza KSB (2018) DDTs-induced antioxidant responses in plants and their influence on the phytoremediation process. Ecotoxicol Environ Saf 147:151–156
Montillet JL, Chamnongpol S, Rustérucci C, Dat J, BVD C, Agnel JP, Battesti C, Inze D, Breusegem FV, Triantaphylide C (2005) Fatty acid hydroperoxides and H2O2 in the execution of hypersensitive cell death in tobacco leaves. Plant Physiol 138:1516–1526
O'Keefe DH, Wiese TE, Brummet SR, Miller TW (1987) Uptake and metabolism of phenolic compounds by the water hyacinth (Eichhornia crassipes). Rec Adv Phytochem 21:101–129
Orton F, Lutz I, Kloas W, Routledge EJ (2009) Endocrine-disrupting effects of herbicides and pentachlorophenol: in vitro and in vivo evidence. Environ Sci Technol 43:2144–2150
Palacio SM, Espinoza-Quiñones FR, Galante RM (2005) Correlation between heavy metal ions (copper, zinc, lead) concentrations and root length of Allium cepa L. in polluted river water. Braz Arch Biol Technol 48:191–196
Patil BC, Bha GI (1992) A comparative study of MH and EMS in the induction of chromosomal aberrations on lateral root meristem in Clitoria ternatea L. Cytologia. 57:259–264
Plewa MJ, Muellner MG, Richardson SD, Fasano KM, Buettner F, Woo YT, McKague AB, Wagner ED (2008) Occurrence, synthesis, and mammalian cell cytotoxicity and genotoxicity of haloacetamides: an emerging class of nitrogenous drinking water disinfection byproducts. Environ Sci Technol 42(3):955–961
Plewa MJ, Wagner ED, Jazwierska P, Richardson SD, Chen PH, McKague AB (2004) Halonitromethane drinking water disinfection byproducts: chemical characterization and mammalian cell cytotoxicity and genotoxicity. Environ Sci Technol. 38(1):62–68
Plewa MJ, Wagner ED, Muellner MG, Hsu KM, Richardson SD (2007) Comparative mammalian cell toxicity of N-DBPs and C-DBPs. In: Karanfil T, Krasner SW, Westerhoff P, Xie Y. Occurrence, formation, health effects and control of disinfection by- products in drinking water. Am Chem Soc 995:36–50
Plewa MJ, Wagner ED, Richardson SD, Thruston AD, Woo YT, McKague AB (2004) Chemical and biological characterization of newly discovered iodoacid drinking water disinfection byproducts. Environ Sci Technol. 38(18):4713–4722
Proudfoot AT (2003) Pentachlorophenol poisoning. Toxicol Rev 22(1):3–11. https://doi.org/10.2165/00139709-200322010-00002
Ranjan J, Mandal DD, Mandal T (2019) Environmental risk appraisement of disinfection byproducts (DBPs) in plant model system: Allium cepa. Environ Sci Pollution Res 26:8609–8622
Repetto G, Jos A, Hazen M, Molero M, Del Peso A, Salguero M, Castillo PD, Rodríguez-Vicente M, Repetto M, (2001) A test battery for the ecotoxicological evaluation of pentachlorophenol. Toxicol. In Vitro 15: 503–509
Richardson SD (1998) Drinking water disinfection by-products. Encyclop Environ Anal Remed 3:1398–1421
Roa S, Patil P (2012) In vitro selection of salt tolerant calli lines and regeneration of salt tolerant Plantlets in Mung Bean (Vigna radiata L. Wilczek). Biotechnology- Molecular Studies and Novel Applications for improved quality of human life 12:197–212
Rodríguez YA, Christofoletti CA, Pedro J, Bueno OC, Malaspina O, Ferreira RAC, Fontanetti CS (2015)Allium cepa and Tradescantia pallida bioassays to evaluate effects of the insecticide imidacloprid. Chemosphere 120:438–442
Sairam RK, Deshmukh PS, Saxena DC (1998) Role of antioxidant systems in wheat genotypes tolerance to water stress. Biol Plant 41:387–394
Saxena S, Joshi P, Grimm B, Arora S (2011) Alleviation of ultraviolet-C-induced oxidative damage through overexpression of cytosolic ascorbate peroxidase. Biologia. 66(6). https://doi.org/10.2478/s11756-011-0120-4
Sharma HA, Barber JT, Ensley HE, Polito MA (1997) A comparison of the toxicity and metabolism of phenol and chlorinated phenols by Lemna gibba, with special reference to 2,4,5-trichlorophenol. Environ Toxicol Chem 16(2):346–350
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot 217037:26
Silveira L, Lima MGF, Reis GB, Palmieri MJ, Andrade-Vieria IF (2017) Toxic effect of environmental pollutants: comparative investigation using Allium cepa L. and Lactuca sativa L. Chemosphere 178:359–367
Singh H, Batish D, Kohli R, Arora K (2007) Arsenic-induced root growth inhibition in mung bean (Phaseolus aureus Roxb.) is due to oxidative stress resulting from enhance lipid peroxidation. Plant Growth Regul 53:65–73
Sponza DT (2003) Application of toxicity tests into discharges of the pulp-paper industry in Turkey. Ecotoxicol Environ Saf 54(1):74–86
Stangroom SJ, Collins CD, Lester JN (1998) Sources of organic micropollutants to lowland rivers. Environ Technol 19:643–666
Stoeva N, Berova M, Zlatev Z (2005) Effect of arsenic on some physiological parameters in bean plants. Biol Plant 49:293–296
Stone JR, Yang S (2006) Hydrogen peroxide: a signaling messenger. Antioxidant Redox Signal 8:243–270
Subramani A, Sundaramurthy P, Lakshmanaperumalsamy AS (1995) Effect of distillery effluent on growth, yield, and productivity of Vigna radiata. Pollut Res 14:477–481
Teixeira A, Morfim MP, Cordova CAS, Charão CCT, Lima VR, Creczynski-Pasa TB (2003) Melatonin protects against pro-oxidant enzymes and reduces lipid peroxidation in distinct membranes induced by the hydroxyl and ascorbyl radicals and by peroxynitrite. J Pineal Res 35(4):262–268
Thiebaut F, Hemerly AS, Ferreira PCG (2019) A role for epigenetic regulation in the adaptation and stress responses of non-model plants. Front Plant Sci 10:246
Tuteja N, Ahmad P, Panda BB, Tuteja R (2009) Genotoxic stress in plants: shedding light on DNA damage, repair and DNA repair helicases. Mutat Res 681:134–149
Vallejo M, Fernández-Castro P, San Román MF, Ortiz I (2015) Assessment of PCDD/fs formation in the Fenton oxidation of 2- chlorophenol: influence of the iron dose applied. Chemosphere 137:135–141
Veningerova M, Prachar V, Uhnak J (1998) Levels of chlorinated phenols in Danube River water. Fresenius Environ Bull 7:224–231
Verma S, Srivastava A (2017) Cytomorphologic parameters in monitoring cytogenotoxic effects of fertilizer in Allium cepa L. Environ Monit Assess 189:159
Wang W, Keturi PH (1990) Comparative seed germination tests using ten plant species for toxicity assessment of a metal engraving effluent sample. Water Air Soil Pollut 52(3–4):369–376
WHO, (2003) Chlorophenols in drinking-water. Background document for preparation of WHO Guidelines for drinking water quality. WHO/SDE/WSH/03.04/47. World Health Organization, Geneva
Yang XD, Dong CJ, Liu JY (2006) A plant mitochondrial phospholipid hydroperoxide glutathione peroxidase: its precise localization and higher enzymatic activity. Plant Mol Biol 62:951–962
Yang M, Zhang X (2016) Current trends in analysis and identification of emerging disinfection byproducts. Trends Environ Anal Chem 10:24–34
Zha JM, Wang ZJ, Schlenk D (2006) Effects of pentachlorophenol on the reproduction of Japanese medaka (Oryzias latipes). Chem Biol Interact 161:26–36
Acknowledgements
The authors would like to acknowledge TEQIP II, MHRD, and the National Institute of Technology, Durgapur for availing good research facilities.
Funding
This work was supported by Prof. Tamal Mandal, Centre for Technological Excellence in Water Purification (CTEWP), (No. DST/TM/WTI/WIC/2K17/84(G)), Department of Chemical Engineering, National Institute of Technology, Durgapur, West Bengal, India.
Author information
Authors and Affiliations
Contributions
Jyoti Ranjan, preparation, methodology, investigation, software, and writing (original draft); Vayam Joshi, methodology, investigation, and preparation; Prof. Tamal Mandal, methodology, data curation, preparation, and writing (reviewing and editing); and Prof. Dalia Dasgupta Mandal, conceptualization, methodology, supervision, validation, and writing (reviewing and editing).
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Gangrong Shi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ranjan , J., Joshi , V., Mandal , T. et al. Ecotoxicological risk assessment of pentachlorophenol, an emerging DBP to plants: evaluation of oxidative stress and antioxidant responses. Environ Sci Pollut Res 28, 27954–27965 (2021). https://doi.org/10.1007/s11356-021-12578-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-12578-6