Abstract
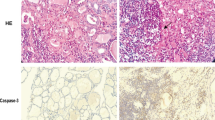
To study the toxicity induced by Nickel sulfate (NiSO4) on thyroid tissue, and investigate the role of apoptosis as the possible mechanism, thirty-two male Wistar rats were randomly divided into control group (normal saline, ip), low dose group (2.5 mg/kg day NiSO4, ip), middle dose group (5 mg/kg day NiSO4, ip), high dose group (10 mg/kg day NiSO4, ip). After 40 consecutive days of treatment, there were obvious pathological changes in the thyroids of high dose group. Free T4 (FT4) and thyroid-stimulating hormone (TSH) were significantly lower in the NiSO4-treated groups than those in the control group (F = 4.992, p = 0.016; F = 4.524, p = 0.012). The mRNA expression of Caspase-3 was significantly higher (F = 10.259, p = 0.014) in all NiSO4-treated groups, and the mRNA expression of Bcl-2 was significantly lower (F = 9.225, p = 0.018) only in the high dose group. Both control group and the NiSO4-treated groups showed no changes in the mRNA expression of Bax gene. The ratio of Bcl-2/Bax decreased with the increase in exposure dose of NiSO4 (F = 13.382, p = 0.015). The mRNA expression of Fas went up in high dose group (F = 66.632, p < 0.001). The Caspase-3, Fas, and the Bax protein expressions measured by immunohistochemistry were consistent with the mRNA expression. The expression of Bcl-2 protein was significantly lower in the test groups than in the control group (F = 3.873, p = 0.025). NiSO4 as an Endocrine Disrupting Chemical may induce the thyroid injury through apoptosis and lead to hypothyroidism. Also, apoptosis in thyroid tissues was closely related to the alternations of Caspase-3, Bcl-2, and Fas mRNA and protein expression.








Similar content being viewed by others
Change history
28 December 2019
The original version of this article unfortunately contained mistakes.
References
Haber L, Erdreicht L, Diamond MA, Ratney R, Zhao Q, Dourson M (2000) Hazard identification and dose response of inhaled nickel-soluble salts. Regul Toxicol Pharmacol 31:210–230
Diagomanolin V, Farhang M, Ghazi-Khansari M, Jafarzadeh N (2004) Heavy metals (Ni, Cr, Cu) in the Karoon waterway river, Iran. Toxicol Lett 151:63–67
Ma F, Feng YJ, Ren N (2003) Environmental biotechnology. Chemical Industry Press, Beijing, pp 124–126
Gao J (2012) Study of ecological risk evaluation of heavy mental pollution at nickel-copper mining area in Jinchang City of Gansu Province. University of South China, Hengyang
Von Burg DR (1999) Toxicology update. J Appl Toxicol 19:379–386
Qian C, Tang W (2006) Advances in research on the relationship between environmental endocrine disruptors and thyroid diseases. J Environmental Hygiene 33(2):106–109
Xu S, He M, Zhong M, Li L, Lu Y, Zhang Y, Zhang L, Yu Z, Zhou Z (2015) The neuroprotective effects of taurine against nickel by reducing oxidative stress and maintaining mitochondrial function in cortical neurons. Neurosci Lett 590:52–57
Gathwan KH, Al-Karkhi IHT, Al-Mulla EAJ (2012) Hepatic toxicity of nickel chloride in mice. Res Chem Intermed 39(6):2537–2542
Scutariu MD, Ciupilan C (2007) Nickel and magnesium effects in the rat kidney, treated with acid retinoic. Comparative study. Rev Med Chir Soc Med Nat Iasi 111(3):744–747
Chen CY, Lin TK, Chang YC, Wang YF, Shyu HW, Lin KH, Chou MC (2010) Nickel (II)-induced oxidative stress,apoptosis, G2/M arrest andgenotoxicity in normal rat kidney cells. J Toxicol Environ Health A 73(8):529–539
Goodman JE, Prueitt RL, Dodge DG, Thakali S (2009) Carcinogenicity assessment of water-soluble nickel compounds. Crit Rev Toxicol 39(5):365–417
Forgacs Z, Massanyi P, Lukac N, Somosy Z (2012) Reproductive toxicology of nickel-review. J Environ Sci Health A 47(9):1249–1260
Duman F, Ozturk F (2010) Nickel accumulation and its effect on biomass, protein content and antioxidative enzymes in roots and leaves of watercress (Nasturtium officinale R Br). J Environ Sci 22(4):526–532
Kasprzak KS, Sunderman FW Jr, Salnikow K (2003) Nickel carcinogenesis. Mutat Res 533(1–2):67–97
Sabir S, Akash MSH, Fiayyaz F, Saleem U, Mehmood MH, Rehman K (2019) Role of cadmium and arsenic as endocrine disruptors in the metabolism of carbohydrates: inserting the association into perspectives. Biomed Pharmacother 114:108802. https://doi.org/10.1016/j.biopha.2019.108802
Wang Q, Yang H, Yang M, Yu Y, Yan M, Zhou L, Liu X, Xiao S, Yang Y, Wang Y, Zheng L, Zhao H, Li Y (2019) Toxic effects of bisphenol A on goldfish gonad development and the possible pathway of BPA disturbance in female and male fish reproduction. Chemosphere 221:235–245
Adedara IA, Awogbindin IO, Adesina AA, Oyebiyi OO, Lawal TA, Farombi EO (2015) Municipal landfill leachate-induced testicular oxidative damage is associated with biometal accumulation and endocrine disruption in rats. Arch Environ Contam Toxicol 68(1):74–82
Souza-Talarico JN, Suchecki D, Juster RP, Plusquellec P, Barbosa Junior F, Bunscheit V, Marcourakis T, de Matos TM, Lupien SJ (2017) Lead exposure is related to hypercortisolemic profiles and allostatic load in Brazilian older adults. Environ Res 154:261–268
Maqbool F, Bahadar H, Niaz K, Baeeri M, Rahimifard M, Navaei-Nigjeh M, Ghasemi-Niri SF (2016) Abdollahi M. Effects of methyl mercury on the activity and gene expression of mouse Langerhans islets and glucose metabolism. Food Chem Toxicol 93:119–128
Wang C, Liang G, Chai L, Wang H (2016) Effects of copper on growth, metamorphosis and endocrine disruption of Bufo gargarizans larvae. Aquat Toxicol 170:24–30
Cempel M, Nikel G (2006) Nickel: a review of its sources and environmental toxicology. Pol J Environ Stud 15:375–382
Liu X, Xiang L, Shao J (2004) The molcular mechanisms of apoptosis induced by heavy metals. Chin J Cell Biol 26(3):235–240
Guo H, Chen L, Cui H, Peng X, Fang J, Zuo Z, Deng J, Wang X, Wu B (2016) Research advances on pathways of nickel-induced apoptosis. Int J Mol Sci 17(1):10
Su L, Deng Y, Zhang Y, Li C, Zhang R, Sun Y, Zhang K, Li J, Yao S (2011) Protective effects of grape seed procyanidin extract against NiSO4-induced apoptosis and oxidative stress in rat testes. Toxicol Mech Methods 21(6):487–494
Zheng GH, Liu CM, Sun JM, Feng ZJ, Cheng C (2014) Nickel-induced oxidative stress and apoptosis in Carassius auratus liver by JNK pathway. Aquat Toxicol 147:105–111
Liu CM, Zheng GH, Ming QL, Chao C, Sun JM (2013) Sesamin protects mouse liver against nickel-induced oxidative DNA damage and apoptosis by the PI3K-Akt pathway. Agric Food Chem 61(5):1146–1154
Guo H, Cui H, Peng X, Fang J, Zuo Z, Deng J, Wang X et al (2014) Modulation of P13K/Akt pathway and Bcl-2 family proteins involved in chicken’s tubular apoptosis induced by nickel chloride (NiCl2). Int J Mol Sci 5(16):22989–23011
Chung SM, Moon JS, Yoon JS, Won KC, Lee HW (2019) Sex-specific effects of blood cadmium on thyroid hormones and thyroid function status: Korean nationwide cross-sectional study. J Trace Elem Med Biol 5(53):55–61
Dolgova NV, Nehzati S, MacDonald TC, Summers KL, Crawford AM, Krone PH, George GN, Pickering IJ (2019) Disruption of selenium transport and function is a major contributor to mercury toxicity in zebrafish larvae. Metallomics 11(3):621–631
Zadjali SA, Nemmar A, Fahim MA, Azimullah S, Subramanian D, Yasin J, Amir N, Hasan MY, Adem A (2015) Lead exposure causes thyroid abnormalities in diabetic rats. Int J Clin Exp Med 8(5):7160–7167
Jain RB, Choi YS (2016) Interacting effects of selected trace and toxic metals on thyroid function. Int J Environ Health Res 26(1):75–91
Andersen H, Larsen S, Spliid H, Christensen ND (1999) Multivariate statistical analysis of organ weights in toxicity studies. Toxicology 136:67–77
Cheng W, Yin C (1992) Effects of nickel chloride (NiCl2) and nickel sulfate (NiSO4) on serum T3, T4 and TSH levels in rats. Journal of Shijiazhuang Medical College (3):4–7
Guyot R, Chatonnet F, Gillet B et al (2014) Toxicogenomic analysis of the ability of brominated flame retardants TBBPA and BDE-209 to disrupt thyroid hormone signaling in neural cells. Toxicology 325:125–132
Parker K, Sunderman FW Jr (1974) Distribution of 63Ni in rabbit tissues following intravenous injection of 63NiCl2. Res Commun Chem Pathol Pharmacol 7(4):755–762
Xie Y, Jing SI, Wang YP et al (2018) E2Fisinvolvedinradioresistanceof carbon ion induced apoptosis via Bax/caspase3 signal pathwayinhuman hepatoma cell. J Cell Physiol 233(2):1312–1320
Chen M, Zhou B, Zhong P, Rajamanickam V, Dai X, Karvannan K, Zhou H, Zhang X, Liang G (2017) Increased intracellular reactive oxygen species mediates the anti-cancer effects of WZ35 via activating mitochondrial apoptosis pathway in prostate cancer cells. Prostate 77(5):489–504
Acknowledgments
The authors thank all those who participated in this study.
Funding
This study was supported by the Program for National Natural Science Foundation of China (31670518).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Our experiments involving the use of rats and all experimental procedures involving animals were approved by The Second Hospital of Lanzhou University Animal Care and Use Committee.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, Y., Chen, H., Zhang, L. et al. The Association Between Thyroid Injury and Apoptosis, and Alterations of Bax, Bcl-2, and Caspase-3 mRNA/Protein Expression Induced by Nickel Sulfate in Wistar Rats. Biol Trace Elem Res 195, 159–168 (2020). https://doi.org/10.1007/s12011-019-01825-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-01825-0