Abstract
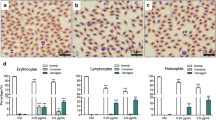
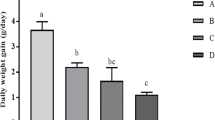
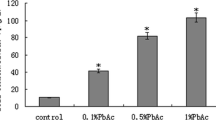
In the current study, we investigated the effect of lead chloride (PbCl2) administration (50 and 100 ppm) on organ and body weight as well as its bioaccumulation during pregnancy and the postnatal period in mice. We showed that lead has no effect on the body weight of mice. However, spleen weight is affected by the two doses of PbCl2 while liver and kidney weights are altered only by the 100-ppm dose. Inductively coupled plasma atomic emission spectrometry (ICP-AES) analysis showed that lead accumulates in the blood, spleen, and thymus. Both doses of PbCl2 significantly reduced splenocyte and thymocyte cell counts after stimulation with lipopolysaccharide (LPS) and phytohemagglutinin A (PHA), respectively. On the other hand, we showed that the levels of Th1 cytokines (interleukin-2 (IL-2), interferon gamma (IFN-γ)), and tumor necrosis factor alpha (TNF-α) were reduced in the serum of mice treated with PbCl2 in a dose-dependent manner, as measured by ELISA. The levels of interleukin-4 (IL-4) and interleukin-10 (IL-10) were very low in untreated mice and were also reduced by treatment with PbCl2. The levels of IL-2, IFN-γ, IL-4, IL-10, and TNF-α secretion differentially decreased in LPS-stimulated splenocytes in lead-treated mice. Using PHA-stimulated thymocytes, we observed a reduction in the levels of IL-2, IL-4, IL-10, and TNF-α in the PbCl2-treated groups. However, IFN-γ concentration in the supernatant of these cells was not decreased when mice were treated with 50 ppm of lead.






Similar content being viewed by others
Abbreviations
- PbCl2 :
-
Lead(II) chloride
- LPS:
-
Lipopolysaccharide
- PHA:
-
Phytohemagglutinin A
- IL-2:
-
Interleukin 2
- IFN-γ:
-
Interferon gamma
- IL-4:
-
Interleukin 4
- IL-10:
-
Interleukin 10
- TNF-α:
-
Tumor necrosis factor alpha
References
Boskabady M, Marefati N, Farkhondeh T et al (2018) The effect of environmental lead exposure on human health and the contribution of inflammatory mechanisms, a review. Environ Int 120:404–420. https://doi.org/10.1016/j.envint.2018.08.013
World Health Organization (2010) Action is needed on chemicals of major public health concern. 20 Avenue Appia, CH-1211 Geneva-27, Switzerland. https://www.who.int/ipcs/features/10chemicals_en.pdf?ua=1
Lawrence DA (1981) In vivo and in vitro effects of lead on humoral and cell-mediated immunity. 31:9
Yücesoy B, Turhan A, Üre M et al (1997) Effects of occupational lead and cadmium exposure on some immunoregulatory cytokine levels in man. Toxicology 123:143–147. https://doi.org/10.1016/S0300-483X(97)00107-8
Heo Y, Lee WT, Lawrence DA (1998) Differential effects of lead and cAMP on development and activities of Th1- and Th2-lymphocytes 1. Toxicol Sci 43:172–185
Queiroz MLS, Almeida M, Gallão MI, Höehr NF (1993) Defective neutrophil function in workers occupationally exposed to lead. Pharmacol Toxicol 72:73–77. https://doi.org/10.1111/j.1600-0773.1993.tb00293.x
McCabe MJ, Lawrence DA (1990) The heavy metal lead exhibits B cell-stimulatory factor activity by enhancing B cell Ia expression and differentiation. J Immunol 145:8
Singh VK, Mishra KP, Rani R et al (2003) Immunomodulation by lead. Immunol Res 28:151–166. https://doi.org/10.1385/IR:28:2:151
Ikenaka Y, Nakayama SMM, Muroya T et al (2012) Effects of environmental lead contamination on cattle in a lead/zinc mining area: changes in cattle immune systems on exposure to lead in vivo and in vitro. Environ Toxicol Chem 31:2300–2305. https://doi.org/10.1002/etc.1951
Dietert RR, Piepenbrink MS (2006) Lead and immune function. Crit Rev Toxicol 36:359–385. https://doi.org/10.1080/10408440500534297
Bussolaro D, Filipak Neto F, Gargioni R et al (2008) The immune response of peritoneal macrophages due to exposure to inorganic lead in the house mouse Mus musculus. Toxicol in Vitro 22:254–260. https://doi.org/10.1016/j.tiv.2007.09.003
Vallverdú-Coll N, Mateo R, Mougeot F, Ortiz-Santaliestra ME (2019) Immunotoxic effects of lead on birds. Sci Total Environ 689:505–515. https://doi.org/10.1016/j.scitotenv.2019.06.251
Goebel C, Flohé SB, Kirchhoff K et al (2000) Orally administered lead chloride induces bias of mucosal immunity. Cytokine 12:1414–1418. https://doi.org/10.1006/cyto.2000.0708
Miller TE, Golemboski KA, Ha RS et al (1998) Developmental exposure to lead causes persistent immunotoxicity in Fischer 344 rats. Toxicol Sci 42:129–135
Heo Y, Parsons PJ, Lawrence DA (1996) Lead differentially modifies cytokine production in vitro and in vivo. Toxicol Appl Pharmacol 138:149–157. https://doi.org/10.1006/taap.1996.0108
Gao D, Kasten-Jolly J, Lawrence DA (2006) The paradoxical effects of lead in interferon-gamma knockout BALB/c mice. Toxicol Sci 89:444–453. https://doi.org/10.1093/toxsci/kfj043
Sarasua SM, Vogt RF, Henderson LO et al (2010) Serum immunoglobulins and lymphocyte subset distributions in children and adults living in communities assessed for lead and cadmium exposure. J Toxicol Environ Health A 60:1–15
Hemdan NYA, Emmrich F, Adham K et al (2005) Dose-dependent modulation of the in vitro cytokine production of human immune competent cells by lead salts. Toxicol Sci 86:75–83. https://doi.org/10.1093/toxsci/kfi177
Fernandez-Cabezudo MJ, Hasan MY, Mustafa N et al (2003) Alpha tocopherol protects against immunosuppressive and immunotoxic effects of lead. Free Radic Res 37:437–445. https://doi.org/10.1080/1071576031000076277
Lee J-E, Dietert RR (2003) Developmental immunotoxicity of lead: impact on thymic function. Birth Defects Res A Clin Mol Teratol 67:861–867. https://doi.org/10.1002/bdra.10092
Snyder JE, Filipov NM, Parsons PJ, Lawrence DA (2000) The efficiency of maternal transfer of lead and its influence on plasma IgE and splenic cellularity of mice. Toxicol Sci 57:87–94. https://doi.org/10.1093/toxsci/57.1.87
Aisemberg J, Vercelli CA, Bariani MV et al (2013) Progesterone is essential for protecting against LPS-induced pregnancy loss. LIF as a potential mediator of the anti-inflammatory effect of progesterone. PLoS ONE 8:e56161. https://doi.org/10.1371/journal.pone.0056161
Nguyen AT, Mandard S, Dray C et al (2014) Lipopolysaccharides-mediated increase in glucose-stimulated insulin secretion: involvement of the GLP-1 pathway. Diabetes 63:471–482. https://doi.org/10.2337/db13-0903
Grara N, Boucenna M, Atailia A et al (2012) Etude expérimentale de la bioaccumulation des éléments traces métalliques Cd/Cu/Zn et Pb chez l’escargot Helix aspersa. Bull L’Institut Sci Rabat 2:183–187
Graham DL, Grace CE, Braun AA et al (2011) Effects of developmental stress and lead (Pb) on corticosterone after chronic and acute stress, brain monoamines, and blood Pb levels in rats. Int J Dev Neurosci 29:45–55. https://doi.org/10.1016/j.ijdevneu.2010.09.008
Hillam RP, Ozkan AN (1986) Comparison of local and systemic immunity after intratracheal, intraperitoneal, and intravenous immunization of mice exposed to either aerosolized or ingested lead. Environ Res 39:265–277. https://doi.org/10.1016/S0013-9351(86)80053-6
Salman NM (2017) Accumulation of lead in the tissues and effects on growth rate of freshwater Cyprinus carpio. J Entomol Zool Stud 5:1499–1502
Fowler BA, Kimmel CA, Woods JS et al (1980) Chronic low-level lead toxicity in the rat. Toxicol Appl Pharmacol 56:59–77
Raza B, Javed M, Ambreen F, Latif F (2016) Toxic effect of lead chloride on antioxidant enzyme in the liver and kidney of fish. J Bioresour Manag 3:9
Cretacci Y, Parsons PJ (2010) Localized accumulation of lead within and among bones from lead-dosed goats. Environ Res 110:26–32. https://doi.org/10.1016/j.envres.2009.09.005
Simons TJB (1984) Active transport of lead by human red blood cells. FEBS Letters 172 (2):250–254
Simons TJB (1986) Passive transport and binding of lead by human red blood cells. J Physiol 378:267–286. https://doi.org/10.1113/jphysiol.1986.sp016219
Sousa RA de, Sabarense CM, Prado GLP, et al (2013) Lead biomonitoring in different organs of lead intoxicated rats employing GF AAS and different sample preparations. Talanta 104:90–96. https://doi.org/10.1016/j.talanta.2012.11.043
TüRkay M, TüRker H, GüVen T (2015) Ultrastructural effects of lead acetate on the spleen of rats. Turk J Biol 39:511–516. https://doi.org/10.3906/biy-1404-48
Barry PS (1981) Concentrations of Lead in the Tissues of Children. Br J Ind Med 38:61–71
Mebius RE, Kraal G (2005) Structure and function of the spleen. Nat Rev Immunol 5:606–616. https://doi.org/10.1038/nri1669
Kasten-Jolly J, Heo Y, Lawrence DA (2010) Impact of developmental lead exposure on splenic factors. ToxicolApplPharmacol 247:105–115. https://doi.org/10.1016/j.taap.2010.06.003
Steffensen I-L, Mesna OJ, Andruchow E, et al (1994) Cytotoxicity and accumulation of Hg, Ag, Cd, Cu, Pb and Zn in human peripheral T and B lymphocytes and monocytes In Vitro. Gen PharmacolVascSyst 25:1621–1633. https://doi.org/10.1016/0306-3623(94)90364-6
Sharma R, Kantwa SM (2011) Effects of Vitamin C on Lead Induced Developing Thymus in Mice: A review. Univers J Environ Res Technol 1:91–102
Fenga C, Gangemi S, Di Salvatore V, et al (2017) Immunological effects of occupational exposure to lead. Mol Med Rep 15:3355–3360. https://doi.org/10.3892/mmr.2017.6381
Iavicoli I, Carelli G, Stanek EJ, et al (2006) Below background levels of blood lead impact cytokine levels in male and female mice. ToxicolApplPharmacol 210:94–99. https://doi.org/10.1016/j.taap.2005.09.016
Rapisarda V, Ledda C, Ferrante M, et al (2016) Blood pressure and occupational exposure to noise and lead (Pb): a cross-sectional study. ToxicolInd Health 32:1729–1736. https://doi.org/10.1177/0748233715576616
Sen A, Heredia N, Senut M-C, et al (2015) Multigenerational epigenetic inheritance in humans: DNA methylation changes associated with maternal exposure to lead can be transmitted to the grandchildren. Sci Rep 5:1–10. https://doi.org/10.1038/srep14466
McCabe MJ, Lawrence DA (1991) Lead, a major environmental pollutant, is immunomodulatory by its differential effects on CD4+ T cell subsets. ToxicolApplPharmacol 111:13–23. https://doi.org/10.1016/0041-008X(91)90129-3
Tian L, Lawrence DA (1995) Lead inhibits nitric oxide production in vitro by murine splenic macrophages. ToxicolApplPharmacol 132:156–163. https://doi.org/10.1006/taap.1995.1096
Valentino M, Rapisarda V, Santarelli L, et al (2007) Effect of lead on the levels of some immunoregulatory cytokines in occupationally exposed workers. Hum ExpToxicol 26:551–556. https://doi.org/10.1177/0960327107073817
Dobrakowski M, Boroń M, Czuba ZP, et al (2016) Cytokines related to three major types of cell-mediated immunity in short- and long-term exposures to lead compounds. J Immunotoxicol 13:770–774. https://doi.org/10.1080/1547691X.2016.1184360
Krocova Z, Macela A, Kroca M, Hernychova L (2000) TheImmunomodulatory Effect(s) of Lead and Cadmium on the Cells of Immune System In Vitro. ToxicolIn Vitro 14:33–40
Kamińska T, Filar J, Madej E, et al (1998) Modification of bovine interferon and tumor necrosis factor production by lead in vivo and in vitro. Arch ImmunolTherExp (Warsz) 46:323–328
Flohé SB, Brüggemann J, Herder C, et al Enhanced proinflammatory response to endotoxin after priming of macrophages with lead ions. J LeukocBiol 3:417–424
García-Lestón J, Roma-Torres J, Mayan O, et al (2012) Assessment of Immunotoxicity Parameters in Individuals Occupationally Exposed to Lead. J Toxicol Environ Health A 75:807–818. https://doi.org/10.1080/15287394.2012.690327
Colombo M, Hamelin C, Kouassi E, et al (2004) Differential effects of mercury, lead, and cadmium on IL-2 production by Jurkat T cells. ClinImmunol 111:311–322. https://doi.org/10.1016/j.clim.2004.02.005
Iavicoli I, Marinaccio A, Castellino N, Carelli G (2004) Altered Cytokine Production in Mice Exposed to Lead Acetate. Int J ImmunopatholPharmacol 17:97–102. https://doi.org/10.1177/03946320040170S216
Mishra KP, Rani R, Yadav VS, Naik S (2010) Effect of lead exposure on lymphocyte subsets and activation markers.ImmunopharmacolImmunotoxicol 32:446–449. https://doi.org/10.3109/08923970903503668
Cooper MA, Fehniger TA, Caligiuri MA (2001) The biology of human natural killer-cell subsets. Trends Immunol 22:633–640. https://doi.org/10.1016/S1471-4906(01)02060-9
Fauriat C, Long EO, Ljunggren H-G, Bryceson YT (2010) Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood 115:2167–2176. https://doi.org/10.1182/blood-2009-08-238469
Exon JH, Talcott PA, Koller LD (1985) Effect of Lead, Polychlorinated Biphenyls, and Cyclophosphamide on Rat Natural Killer Cells, lnterleukin 2, and Antibody Synthesis’. FundamApplToxicol 5:158–164
Friberg D, Bryant J, Shannon W, Whiteside TL (1994) In vitro cytokine production by normal human peripheral blood mononuclear cells as a measure of immunocompetence or the state of activation. ClinDiagn Lab Immunol 1:261–268
Luzina IG, Keegan AD, Heller NM, et al (2012) Regulation of inflammation by interleukin-4: a review of “alternatives.” J LeukocBiol 92:753–764. https://doi.org/10.1189/jlb.0412214
Couper KN, Blount DG, Riley EM (2008) IL-10: The Master Regulator of Immunity to Infection. J Immunol 180:5771–5777. https://doi.org/10.4049/jimmunol.180.9.5771
Laing KJ, Wang T, Zou J et al (2001) Cloning and expression analysis of rainbow trout Oncorhynchus mykiss tumour necrosis factor-α: rainbow trout tumour necrosis factor-α. Eur J Biochem 268:1315–1322. https://doi.org/10.1046/j.1432-1327.2001.01996.x
Vassalli P (1992) The pathophysiology of tumor necrosis factors. Annu Rev Immunol 10:411–452
Sengupta M, Bishayi B (2002) Effect of lead and arsenic on murine macrophage response. Drug Chem Toxicol 25:459–472. https://doi.org/10.1081/DCT-120014796
Kerkvliet NI, Baecher-Steppan L (1982) Immunotoxicology studies on lead: effects of exposure on tumor growth and cell-mediated tumor immunity after syngeneic or allogeneic stimulation. Immunopharmacology 4:213–224. https://doi.org/10.1016/0162-3109(82)90003-0
Acknowledgments
The authors gratefully acknowledge the expert technical assistance of Pr. Mohamed EL-Baghdadi (Faculty of Sciences and Techniques, Sultan Moulay Slimane University, Beni Mellal, Morocco) and Dr. Amorette Barber (Associate Professor of Biology, Longwood University, Farmville, VA, USA), for reading and editing the manuscript.
Funding
This work was supported by the “Foundation Lalla Salma: Cancer Prevention and Treatment” Rabat. Morocco. Project 9/2013.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ajouaoi, S., Bouchmaa, N., Idir, A. et al. Treatment with Lead Chloride During Pregnancy and the Postnatal Period Alters Cell Proliferation and Immune Function in Swiss Albino Mice. Biol Trace Elem Res 196, 195–203 (2020). https://doi.org/10.1007/s12011-019-01917-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-01917-x