Abstract
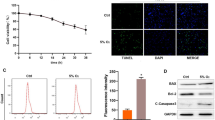
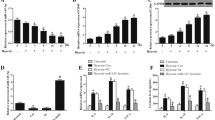
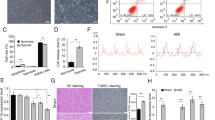
This study aimed to investigate the mechanism of how miR-362-3p/orosomucoid 1 (ORM1) involved in hypoxia/reoxygenation (H/R)-induced cardiomyocytes injury. Based on data obtained from Gene Expression Omnibus (GEO) database, we revealed that ORM1 was highly expressed and positively correlated with the expression of inflammatory factors (MAPK1, MAPK3, IL1B and CASP9). miR-362-3p was identified as an upstream regulatory miRNA of ORM1 and negatively modulated the mRNA and protein expression levels of ORM1 in H/R-injured cardiomyocytes. Moreover, we found that miR-362-3p was downregulated in cardiomyocytes injured by H/R. The promoting influence of miR-362-3p mimic on the proliferation and the inhibitory effect of miR-362-3p mimic on the apoptosis of H/R-stimulated cardiomyocytes were eliminated by overexpression of ORM1. Furthermore, miR-362-3p affected the expression of MAPK1, MAPK3, IL1B and CASP9 in H/R-injured cardiomyocytes through targeting ORM1. Our outcomes illustrated that miR-362-3p exhibited a protective influence on H/R-induced cardiomyocytes through targeting ORM1.





Similar content being viewed by others
Data Availability
The data and material are available from the corresponding author on reasonable request.
Abbreviations
- ORM1:
-
Orosomucoid 1
- miR-362-3p:
-
MicroRNA-362-3p
- AMI:
-
Acute myocardial infarction
- H/R:
-
Hypoxia/reoxygenation
- WHO:
-
World Health Organization
- CVD:
-
Cardiovascular disease
- MiRNAs:
-
MicroRNAs
- MAPK1/3:
-
Mitogen-activated protein kinase 1/3
- ERKs:
-
MAP kinase
References
Mendis, S. (2017). Global progress in prevention of cardiovascular disease. Cardiovasc Diagn Ther, 7(Suppl 1), S32–S38.
Almamari, R. S. S., Muliira, J. K., & Lazarus, E. R. (2019). Self-reported sleep quality and depression in post myocardial infarction patients attending cardiology outpatient clinics in Oman. Int J Nurs Sci, 6(4), 371–377.
Cong, X. Q., et al. (2015). Short-Term Effect of Autologous Bone Marrow Stem Cells to Treat Acute Myocardial Infarction: A Meta-Analysis of Randomized Controlled Clinical Trials. J Cardiovasc Transl Res, 8(4), 221–231.
Shinde, A. V., & Frangogiannis, N. G. (2014). Fibroblasts in myocardial infarction: a role in inflammation and repair. Journal of Molecular and Cellular Cardiology, 70, 74–82.
Eghbalzadeh, K., et al. (2019). Compromised Anti-inflammatory Action of Neutrophil Extracellular Traps in PAD4-Deficient Mice Contributes to Aggravated Acute Inflammation After Myocardial Infarction. Front Immunol, 10, 2313.
Ceciliani, F., et al. (2012). Acute phase proteins in ruminants. J Proteomics, 75(14), 4207–4231.
Li, F., et al. (2016). The increased excretion of urinary orosomucoid 1 as a useful biomarker for bladder cancer. American Journal of Cancer Research, 6(2), 331–340.
Chihara, D., et al. (2016). Management strategies and outcomes for very elderly patients with diffuse large B-cell lymphoma. Cancer, 122(20), 3145–3151.
McGuckin, M. M., et al. (2020). The acute phase protein orosomucoid 1 is upregulated in early lactation but does not trigger appetite-suppressing STAT3 signaling via the leptin receptor. Journal of Dairy Science, 103(5), 4765–4776.
Sai, K., et al. (2014). Distal promoter regions are responsible for differential regulation of human orosomucoid-1 and -2 gene expression and acute phase responses. Biological &/and Pharmaceutical Bulletin, 37(1), 164–168.
Chen, W. Q., et al. (2013). Polymorphism of ORM1 is associated with the pharmacokinetics of telmisartan. PLoS ONE, 8(8), e70341.
Zhou, Z., et al. (2016). S100A9 and ORM1 serve as predictors of therapeutic response and prognostic factors in advanced extranodal NK/T cell lymphoma patients treated with pegaspargase/gemcitabine. Sci Rep, 6, 23695.
Giovanni, L., et al. (2012). The Acute Phase Reactant Orosomucoid-1 Is a Bimodal Regulator of Angiogenesis with Time- and Context-Dependent Inhibitory and Stimulatory Properties. PLoS ONE. https://doi.org/10.1371/journal.pone.0041387.
Cai, L., et al. (2016). ORMDL proteins regulate ceramide levels during sterile inflammation. Journal of Lipid Research, 57(8), 1412–1422.
Lee, E. G., et al. (2016). Tolerogenic dendritic cells show gene expression profiles that are different from those of immunogenic dendritic cells in DBA/1 mice. Autoimmunity, 49(2), 90–101.
Astrup, L. B., et al. (2019). Staphylococcus aureus infected embolic stroke upregulates Orm1 and Cxcl2 in a rat model of septic stroke pathology. Neurological Research, 41(5), 399–412.
Paraskevi, A., et al. (2012). Circulating MicroRNA in inflammatory bowel disease. Journal of Crohn’s and Colitis, 6(9), 900–904.
Jiang, Z. H., et al. (2019). miRNA342 suppresses renal interstitial fibrosis in diabetic nephropathy by targeting SOX6. International Jouney of Molecular Medicine. https://doi.org/10.3892/ijmm.2019.4388.
Sun, T., et al. (2017). The Role of MicroRNAs in Myocardial Infarction: From Molecular Mechanism to Clinical Application. International Journal of Molecular Sciences. https://doi.org/10.3390/ijms18040745.
Zhang, J., et al. (2019). Upregulating MicroRNA-203 Alleviates Myocardial Remodeling and Cell Apoptosis Through Downregulating Protein Tyrosine Phosphatase 1B in Rats With Myocardial Infarction. Journal of Cardiovascular Pharmacology, 74(5), 474–481.
Boon, R. A., & Dimmeler, S. (2015). MicroRNAs in myocardial infarction. Nat Rev Cardiol, 12(3), 135–142.
Mirna, M., et al. (2019). MicroRNAs in Inflammatory Heart Diseases and Sepsis-Induced Cardiac Dysfunction: A Potential Scope for the Future? Cells. https://doi.org/10.3390/cells8111352.
Wang, N., et al. (2018). miR-362-3p regulates cell proliferation, migration and invasion of trophoblastic cells under hypoxia through targeting Pax3. Biomedicine & Pharmacotherapy, 99, 462–468.
Assiri, A. A., et al. (2019). MicroRNA 362–3p Reduces hERG-related Current and Inhibits Breast Cancer Cells Proliferation. Cancer Genomics & Proteomics, 16(6), 433–442.
Omidbakhsh, A., et al. (2018). Micro-RNAs -106a and -362-3p in Peripheral Blood of Inflammatory Bowel Disease Patients. Open Biochemistry Journal, 12, 78–86.
Zhang, H. J., Zhang, Y. N., & Teng, Z. Y. (2019). Downregulation of miR-16 protects H9c2(2–1) cells against hypoxia/reoxygenation damage by targeting CIAPIN1 and regulating the NF-κB pathway. Mol Med Rep, 20(4), 3113–3122.
Liu, D. Z., et al. (2010). Brain and blood microRNA expression profiling of ischemic stroke, intracerebral hemorrhage, and kainate seizures. Journal of Cerebral Blood Flow and Metabolism, 30(1), 92–101.
Kiss, A., et al. (2020). MicroRNA Expression Profile Changes after Cardiopulmonary Bypass and Ischemia/Reperfusion-Injury in a Porcine Model of Cardioplegic Arrest. Diagnostics (Basel). https://doi.org/10.3390/diagnostics10040240.
Díaz, I., et al. (2017). miR-125a, miR-139 and miR-324 contribute to Urocortin protection against myocardial ischemia-reperfusion injury. Scientific Reports. https://doi.org/10.1038/s41598-017-09198-x.
Africander, D., Louw, R., & Hapgood, J. P. (2013). Investigating the anti-mineralocorticoid properties of synthetic progestins used in hormone therapy. Biochemical and Biophysical Research Communications, 433(3), 305–310.
Wan, J. J., et al. (2019). Role of acute-phase protein ORM in a mice model of ischemic stroke. Journal of Cellular Physiology, 234(11), 20533–20545.
Nemeth, B., et al. (2019). Urinary orosomucoid: a new marker of cardiovascular risk in psoriatic patients? Therapeutics and Clinical Risk Management, 15, 831–837.
Araszkiewicz, A., et al. (2013). The impact of ischemia-reperfusion injury on the effectiveness of primary angioplasty in ST-segment elevation myocardial infarction. Postepy Kardiol Interwencyjnej, 9(3), 275–281.
Chen, Q., et al. (2016). Up-regulation of miRNA-221 inhibits hypoxia/reoxygenation-induced autophagy through the DDIT4/mTORC1 and Tp53inp1/p62 pathways. Biochemical and Biophysical Research Communications, 474(1), 168–174.
Fan, Z. X., & Yang, J. (2015). The role of microRNAs in regulating myocardial ischemia reperfusion injury. Saudi Medical Journal, 36(7), 787–793.
Ren, X. P., et al. (2009). MicroRNA-320 is involved in the regulation of cardiac ischemia/reperfusion injury by targeting heat-shock protein 20. Circulation, 119(17), 2357–2366.
Liang, Y. P., et al. (2019). The lncRNA ROR/miR-124–3p/TRAF6 axis regulated the ischaemia reperfusion injury-induced inflammatory response in human cardiac myocytes. Journal of Bioenergetics and Biomembranes. https://doi.org/10.1007/s10863-019-09812-9.
Qian, W., et al. (2014). Protective effect of tetramethylpyrazine on myocardial ischemia-reperfusion injury. Evid Based Complement Alternat Med, 2014, 107501.
Liu, J. Y., et al. (2019). Protective effect of down-regulated microRNA-27a mediating high thoracic epidural block on myocardial ischemia-reperfusion injury in mice through regulating ABCA1 and NF-kappaB signaling pathway. Biomedicine & Pharmacotherapy, 112, 108606.
Sun, W., et al. (2019). Gastrodin ameliorates microvascular reperfusion injury-induced pyroptosis by regulating the NLRP3/caspase-1 pathway. J Physiol Biochem. https://doi.org/10.1007/s13105-019-00702-7.
Stein, J. M., et al. (2009). The role of the composite interleukin-1 genotype in the association between periodontitis and acute myocardial infarction. Journal of Periodontology, 80(7), 1095–1102.
Chen, D.-Q., et al. (2019). Identification of Differentially Expressed Genes and Signaling Pathways in Acute Myocardial Infarction Based on Integrated Bioinformatics Analysis. Cardiovascular therapeutics, 2019, 8490707–8490707.
Jablonski, K. A., et al. (2016). Control of the Inflammatory Macrophage Transcriptional Signature by miR-155. PLoS ONE, 11(7), e0159724.
Wu, L. K., et al. (2016). High levels of glucose promote the activation of hepatic stellate cells via the p38-mitogen-activated protein kinase signal pathway. Genetics and molecular research : GMR. https://doi.org/10.4238/gmr.15038419.
Jung, Y.-C., et al. (2016). Kazinol-E is a specific inhibitor of ERK that suppresses the enrichment of a breast cancer stem-like cell population. Biochemical and biophysical research communications, 470(2), 294–299.
Li, X., et al. (2017). Down-regulation of lncRNA KCNQ1OT1 protects against myocardial ischemia/reperfusion injury following acute myocardial infarction. Biochemical and biophysical research communications, 491(4), 1026–1033.
Chen, Y., et al. (2017). Role of carvacrol in cardioprotection against myocardial ischemia/reperfusion injury in rats through activation of MAPK/ERK and Akt/eNOS signaling pathways. European journal of pharmacology, 796, 90–100.
Choi, Y. W., et al. (2016). MicroRNA Expression Signatures Associated With BRAF-Mutated Versus KRAS-Mutated Colorectal Cancers. Medicine (Baltimore), 95(15), e3321.
Hao, Y.-L., et al. (2018). The role of microRNA-1 targeting of MAPK3 in myocardial ischemia-reperfusion injury in rats undergoing sevoflurane preconditioning via the PI3K/Akt pathway. American journal of physiology. Cell physiology, 315(3), C380–C388.
He, B., et al. (2011). Role of miR-1 and miR-133a in myocardial ischemic postconditioning. Journal of Biomedical Science, 18, 22.
Ligresti, G., et al. (2012). The acute phase reactant orosomucoid-1 is a bimodal regulator of angiogenesis with time- and context-dependent inhibitory and stimulatory properties. PLoS ONE, 7(8), e41387.
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Handling Editor: Dipak K Dube.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shi, M., Ma, X., Yang, Q. et al. miR-362-3p Targets Orosomucoid 1 to Promote Cell Proliferation, Restrain Cell Apoptosis and Thereby Mitigate Hypoxia/Reoxygenation-Induced Cardiomyocytes Injury. Cardiovasc Toxicol 21, 387–398 (2021). https://doi.org/10.1007/s12012-020-09631-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-020-09631-0