Abstract
Background
Little is known about the sequelae of chronic sympathetic nervous system (SNS) activation in patients with pulmonary arterial hypertension (PAH) and right heart failure (RHF). We aimed to, (1) validate the use of [11C]-meta-hydroxyephedrine (HED) for assessing right ventricular (RV) SNS integrity, and (2) determine the effects of β-receptor blockade on ventricular function and myocardial SNS activity in a PAH rat model.
Methods
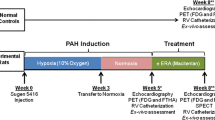
PAH was induced in male Sprague-Dawley rats (N = 36) using the Sugen+chronic hypoxia model. At week 5 post-injection, PAH rats were randomized to carvedilol (15 mg·kg−1·day−1 oral; N = 16) or vehicle (N = 16) for 4 weeks. Myocardial SNS function was assessed with HED positron emission tomography(PET).
Results
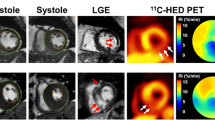
With increasing PAH disease severity, immunohistochemistry confirmed selective sympathetic denervation within the RV and sparing of parasympathetic nerves. These findings were confirmed on PET with a significant negative relationship between HED volume of distribution(DV) and right ventricular systolic pressure (RVSP) in the RV (r = −0.90, p = 0.0003). Carvedilol did not reduce hemodynamic severity compared to vehicle. RV ejection fraction (EF) was lower in both PAH groups compared to control (p < 0.05), and was not further reduced by carvedilol. Carvedilol improved SNS function in the LV with significant increases in the HED DV, and decreased tracer washout in the LV (p < 0.05) but not RV.
Conclusions
PAH disease severity correlated with a reduction in HED DV in the RV. This was associated with selective sympathetic denervation. Late carvedilol treatment did not lead to recovery of RV function. These results support the role of HED imaging in assessing SNS innervation in a failing right ventricle.
Spanish Abstract
Antecedentes
Poco se sabe de las secuelas en la activación del sistema nervioso simpático (SNS) en pacientes con hipertensión arterial pulmonar (HAP) y falla cardíaca derecha (FCD). Nuestros objetivos fueron: (1) validar el uso de la 11C-Meta-hidroxiepinefrina (HED) para evaluar al ventrículo derecho y la integridad del SNS y (2) determinar los efectos del bloqueo de B-receptores en la función ventricular y la actividad miocárdica del SNS en un modelo murino de HAP.
Métodos
Se indujo HAP en modelos murinos Sprague-Dawley machos (n = 36) usando el modelo Sugen-hipoxia crónica. A la semana 5 postinyección, los modelos murinos con HAP fueron randomizados a carvedilol (15mg/kg/día oral n = 16) o vehículo (n = 16) durante 4 semanas. La función miocárdica del SNS fue evaluada con tomografía por emisión de positrones (PET) con HED.
Resultados
Con el incremento de la severidad de la HAP, por inmunohistoquímica se demostró denervación simpática selectiva y preservación de nervios parasimpáticos en el ventrículo derecho. Estos hallazgos fueron confirmados con la PET con una significativa relación inversa entre el volumen de distribución (VD) del HED y la presión sistólica (PSVD) en ventrículo derecho (r = 0.90, p = 0.0003). El Carveridol no redujo la severidad hemodinámica en comparación con el vehículo. La fracción de eyección (FE)del ventrículo derecho fue menor en ambos grupos con PAH comparada con el grupo control (p < 0.05) y no se redujo más con el uso del carveridol. El Carveridol mejoró la función del SNS en el ventrículo derecho con incremento significativo del HED VD, y una disminución en el lavado del radiotrazador por el ventrículo izquierdo (p < 0.05) pero no por el derecho.
Conclusiones
La severidad enfermedad por HAP se correlacionó con una reducción del HED VD en VD. Esto fue asociado con denervación simpática selectiva. El tratamiento tardío con carveridol no condujó a la recuperación de la función del VD. Estos resultados apoyan el rol de la imagen con HED para evaluar la inervación del SNS en el ventrículo derecho en falla.
Chinese Abstract
背景
对于肺动脉高压 (PAH) 和右心衰竭 (RHF) 患者的慢性交感神经系统 (SNS) 激活的后遗症知之甚少。 本文研究目标: 1)验证[11C]-间-羟基麻黄碱 (HED) 用于评估右心室SNS完整性, 2) 确定在PAH大鼠模型中β受体阻滞剂对改善心室功能和心肌SNS活性的作用。
方法
在雄性Sprague-Dawley大鼠 (n = 36) 中使用Sugen +慢性缺氧模型诱导PAH。 在注射后第5周, 将PAH大鼠随机分为卡维地洛组 (15 mg / kg /天, 口服; n = 16) 和溶剂组 (n = 16), 共治疗4周。 采用HED正电子发射断层扫描 (PET)评估心肌SNS功能。
结果
随着PAH病情恶化, 免疫组化证实了右室选择性交感神经去神经化和副交感神经的保留。 这些发现在PET上得到证实, 右室的HED容积分布 (DV) 与右室收缩压 (RVSP) 之间存在显着的负相关 (r = -0.90,p = 0.0003) 。 与溶剂组相比, 卡维地洛组并不能降低血流动力学的严重程度。与对照组相比, 两个PAH组的右心室射血分数 (EF) 均较低(p <.05) , 且并没有因为卡维地洛的使用而进一步降低。左室HED DV显著增加, 提示卡维地洛改善了左心室的SNS功能。左心室的示踪剂洗脱减少 (p <0.05) , 但右心室的洗脱没有减少。
结论
PAH疾病严重程度与右室HED DV降低相关。 这与选择性交感神经去神经化有关。 晚期给予卡维地洛治疗未能使RV功能得到恢复。 这些结果支持了HED成像在评估右心室衰竭中的SNS神经分布中的作用。
French Abstract
Contexte
On sait peu de choses sur les séquelles de l'activation chronique du système nerveux sympathique (SNS) chez les patients souffrant d'hypertension artérielle pulmonaire (HTAP) et d'insuffisance ventriculaire droite (IVD). Nous avons tenté de :1) valider l'utilisation de la [11C] -meta-hydroxyephedrine (MHED) pour évaluer l’intégrité du SNS ventriculaire droit (VD) et 2) déterminer les effets du blocage des récepteurs β sur la fonction ventriculaire et l’activité du SNS myocardique en utilisant un modèle de rat HTAP.
Méthodes
L'HTAP a été induite chez des rats mâles Sprague-Dawley (N = 36) à l'aide du modèle Sugen + hypoxie chronique. À la 5ieme semaine après l'injection, les rats HTAP ont été randomisés pour recevoir du Carvédilol (15 mg / kg / jour par voie orale; n = 16) ou le véhicule (n = 16) pour 4 semaines. La fonction SNS myocardique a été évaluée par tomographie par émission de positons (TEP) avec du MHED marqué au carbone 11.
Résultats
Avec l'augmentation de la sévérité de l'HTAP, l'immunohistochimie a confirmé la dénervation sélective du système nerveux sympathique du VD et l’épargne des nerfs parasympathiques. Ces résultats ont été confirmés sur le PET avec une corrélation négative significative entre le montant de captation de MHED et la pression systolique du ventricule droit (PSVD) (r = -0,90, p = 0,0003). En comparaison avec le véhicule, le Carvédilol n'a pas réduit la sévérité de l’HTAP. La fraction d'éjection du VD s’est avérée inférieure faible dans les deux groupes d’HTAP par rapport au groupe témoin (p <0,05), et n'a pas été réduite davantage par le Carvédilol. Le Carvédilol a amélioré la fonction SNS du ventricule gauche (VG) en parallèle à augmentation significative de la captation de l’MHED et une diminution de la disparition du traceur au niveau du VG (p <0,05) mais pas au niveau du VD
Conclusions
La sévérité de l'HTAP est corrélée à une réduction de la captation de MHED au niveau du VD reflétant sa dénervation sympathique sélective. Un traitement tardif au Carvédilol ne permet pas de récupérer la fonction du VD. Ces résultats supportent le rôle de l'imagerie MHED-TEP dans l'évaluation de l'innervation SNS en cas d’insuffisance du VD.









Similar content being viewed by others
Abbreviations
- DBH:
-
Dopamine-β-hydroxylase
- DV:
-
Volume of distribution
- HED:
-
Hydroxyephedrine
- PAAT:
-
Pulmonary artery acceleration time
- PAH:
-
pulmonary arterial hypertension
- RHF:
-
Right heart failure
- RVSP:
-
Right ventricular systolic pressure
- SNS:
-
Sympathetic nervous system
- VaChT:
-
Vesicular acetylcholine transporter
References
D’Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 191;115:343-9.
Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J. The sympathetic nervous system in heart failure. J Am Coll Cardiol 2009;54:1747-62. https://doi.org/10.1016/j.jacc.2009.05.015.
Ciarka A, Doan V, Velez-Roa S, Naeije R, van de Borne P. Prognostic significance of sympathetic nervous system activation in pulmonary arterial hypertension. Am J Respir Crit Care Med 2010;181:1269-75. https://doi.org/10.1164/rccm.200912-1856OC.
Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med 1996;334:1349-55. https://doi.org/10.1056/NEJM199605233342101.
Galiè N, Humbert M, Vachiery J-L, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS) Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016;37:67-119. https://doi.org/10.1093/eurheartj/ehv317.
Ishikawa M, Sato N, Asai K, Takano T, Mizuno K. Effects of a pure α/β-adrenergic receptor blocker on monocrotaline-induced pulmonary arterial hypertension with right ventricular hypertrophy in rats. Circ J 2009;73:233-41.
Bogaard HJ, Natarajan R, Mizuno S, Abbate A, Chang PJ, Chau VQ, et al. Adrenergic receptor blockade reverses right heart remodeling and dysfunction in pulmonary hypertensive rats. Am J Respir Crit Care Med 2010;182:652-60. https://doi.org/10.1164/rccm.201003-0335OC.
Provencher S, Herve P, Jais X, Lebrec D, Humbert M, Simonneau G, et al. Deleterious effects of β-blockers on exercise capacity and hemodynamics in patients with portopulmonary hypertension. Gastroenterology 2006;130:120-26. https://doi.org/10.1053/j.gastro.2005.10.013.
van Campen JSJA, de Boer K, van de Veerdonk MC, van der Bruggen CEE, Allaart CP, Raijmakers PG, et al. Bisoprolol in idiopathic pulmonary arterial hypertension: An explorative study. Eur Respir J 2016;48:787-96. https://doi.org/10.1183/13993003.00090-2016.
Rubin LJ. The adrenergic nervous system as a therapeutic target in pulmonary arterial hypertension: A cautionary tale. Eur Respir J 2016;48:617-8. https://doi.org/10.1183/13993003.01333-2016.
Poole-Wilson PA, Swedberg K, Cleland JGF, Di Lenarda A, Hanrath P, Komajda M, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet Lond Engl 2003;362:7-13. https://doi.org/10.1016/S0140-6736(03)13800-7.
Farha S, Saygin D, Park MM, Cheong HI, Asosingh K, Comhair SAA, et al. Pulmonary arterial hypertension treatment with carvedilol for heart failure: A randomized controlled trial. JCI Insight 2017. https://doi.org/10.1172/jci.insight.95240.
Lee W, Woo ER, Choi JS. Effects of myricetin on the bioavailability of carvedilol in rats. Pharm Biol 2012;50:516-22. https://doi.org/10.3109/13880209.2011.611141.
Wang T, Wu KY, Miner RC, Renaud JM, Beanlands RSB, deKemp RA. Reproducible quantification of cardiac sympathetic innervation using graphical modeling of carbon-11-meta-hydroxyephedrine kinetics with dynamic PET-CT imaging. EJNMMI Res 2018;8:63. https://doi.org/10.1186/s13550-018-0421-5.
Harms HJ, de Haan S, Knaapen P, Allaart CP, Rijnierse MT, Schuit RC, et al. Quantification of [(11)C]-meta-hydroxyephedrine uptake in human myocardium. EJNMMI Res 2014;4:52. https://doi.org/10.1186/s13550-014-0052-4.
Sisson JC, Bolgos G, Johnson J. Measuring acute changes in adrenergic nerve activity of the heart in the living animal. Am Heart J 1991;121:1119-23. https://doi.org/10.1016/0002-8703(91)90671-4.
Thackeray JT, deKemp RA, Beanlands RS, DaSilva JN. Early diabetes treatment does not prevent sympathetic dysinnervation in the streptozotocin diabetic rat heart. J Nucl Cardiol Off Publ Am Soc Nucl Cardiol 2014;21:829-41. https://doi.org/10.1007/s12350-014-9900-x.
Tipre DN, Fox JJ, Holt DP, Green G, Yu J, Pomper M, et al. In vivo PET imaging of cardiac presynaptic sympathoneuronal mechanisms in the rat. J Nucl Med 2008;49:1189-95. https://doi.org/10.2967/jnumed.107.050252.
Thackeray JT, Renaud JM, Kordos M, Klein R, deKemp RA, Beanlands RSB, et al. Test–retest repeatability of quantitative cardiac 11C-meta-hydroxyephedrine measurements in rats by small animal positron emission tomography. Nucl Med Biol 2013;40:676-81. https://doi.org/10.1016/j.nucmedbio.2013.03.007.
Matsunari I, Aoki H, Nomura Y, Takeda N, Chen W-P, Taki J, et al. Iodine-123 metaiodobenzylguanidine imaging and carbon-11 hydroxyephedrine positron emission tomography compared in patients with left ventricular dysfunction. Circ Cardiovasc Imaging 2010;3:595-603. https://doi.org/10.1161/CIRCIMAGING.109.920538.
Thackeray JT, Renaud JM, Kordos M, Klein R, Dekemp RA, Beanlands RSB, et al. Test-retest repeatability of quantitative cardiac 11C-meta-hydroxyephedrine measurements in rats by small animal positron emission tomography. Nucl Med Biol 2013;40:676-81. https://doi.org/10.1016/j.nucmedbio.2013.03.007.
van den Hoff J, Burchert W, Börner AR, Fricke H, Kühnel G, Meyer GJ, et al. [1-(11)C]Acetate as a quantitative perfusion tracer in myocardial PET. J Nucl Med Off Publ Soc Nucl Med 2001;42:1174-82.
Gomez O, Okumura K, Honjo O, Sun M, Ishii R, Bijnens B, et al. Heart rate reduction improves biventricular function and interactions in experimental pulmonary hypertension. Am J Physiol Heart Circ Physiol 2018;314:H542-51. https://doi.org/10.1152/ajpheart.00493.2017.
de Man FS, Handoko ML, van Ballegoij JJM, Schalij I, Bogaards SJP, Postmus PE, et al. Bisoprolol delays progression towards right heart failure in experimental pulmonary hypertension. Circ Heart Fail 2012;5:97-105. https://doi.org/10.1161/CIRCHEARTFAILURE.111.964494.
Mak S, Witte KK, Al-Hesayen A, Granton JJ, Parker JD. Cardiac sympathetic activation in patients with pulmonary arterial hypertension. AJP Regul Integr Comp Physiol 2012;302:R1153-57. https://doi.org/10.1152/ajpregu.00652.2011.
Kaye DM, Lefkovits J, Jennings GL, Bergin P, Broughton A, Esler MD. Adverse consequences of high sympathetic nervous activity in the failing human heart. J Am Coll Cardiol 1995;26:1257-63. https://doi.org/10.1016/0735-1097(95)00332-0.
Fallavollita JA, Heavey BM, Luisi AJ, Michalek SM, Baldwa S, Mashtare TL, et al. Regional myocardial sympathetic denervation predicts the risk of sudden cardiac arrest in ischemic cardiomyopathy. J Am Coll Cardiol 2014;63:141-9. https://doi.org/10.1016/j.jacc.2013.07.096.
Zelt JGE, deKemp RA, Rotstein BH, Nair GM, Narula J, Ahmadi A, et al. Nuclear imaging of the cardiac sympathetic nervous system: A disease-specific interpretation in heart failure. JACC Cardiovasc Imaging 2019. https://doi.org/10.1016/j.jcmg.2019.01.042.
Yamazaki J, Muto H, Kabano T, Yamashina S, Nanjo S, Inoue A. Evaluation of beta-blocker therapy in patients with dilated cardiomyopathy–Clinical meaning of iodine 123-metaiodobenzylguanidine myocardial single-photon emission computed tomography. Am Heart J 2001;141:645-52. https://doi.org/10.1067/mhj.2001.112783.
Fujimoto S, Inoue A, Hisatake S, Yamashina S, Yamashina H, Nakano H, et al. Usefulness of 123I-metaiodobenzylguanidine myocardial scintigraphy for predicting the effectiveness of beta-blockers in patients with dilated cardiomyopathy from the standpoint of long-term prognosis. Eur J Nucl Med Mol Imaging 2004;31:1356-61. https://doi.org/10.1007/s00259-004-1557-2.
Srivastava D, Olson EN. A genetic blueprint for cardiac development. Nature 2000;407:221-6. https://doi.org/10.1038/35025190.
Oki H, Inoue S, Makishima N, Takeyama Y, Shiokawa A. Cardiac sympathetic innervation in patients with dilated cardiomyopathy: Immunohistochemical study using Anti-tyrosine hydroxylase antibody. Jpn Circ J 1994;58:389-394. https://doi.org/10.1253/jcj.58.389.
Machado CR, Camargos ER, Guerra LB, Maria da Consolação VM (2000) Cardiac autonomic denervation in congestive heart failure: Comparison of Chagas’ heart disease with other dilated cardiomyopathy. Hum Pathol 2000;31:3-10.
Yoshida K, Saku K, Kamada K, Abe K, Tanaka-Ishikawa M, Tohyama T, et al. Electrical vagal nerve stimulation ameliorates pulmonary vascular remodeling and improves survival in rats with severe pulmonary arterial hypertension. JACC Basic Transl Sci 2018;3:657-71. https://doi.org/10.1016/j.jacbts.2018.07.007.
da Silva Gonçalves Bós D, Van Der Bruggen CEE, Kurakula K, Sun X-Q, Casali KR, Casali AG ,et al. Contribution of Impaired Parasympathetic Activity to Right Ventricular Dysfunction and Pulmonary Vascular Remodeling in Pulmonary Arterial Hypertension. Circulation 2018;137:910-24. https://doi.org/10.1161/CIRCULATIONAHA.117.027451.
Yamamoto K, Origasa H, Hori M, J-DHF Investigators. Effects of carvedilol on heart failure with preserved ejection fraction: The Japanese Diastolic Heart Failure Study (J-DHF). Eur J Heart Fail 2013;15:110-8. https://doi.org/10.1093/eurjhf/hfs141.
Suwa M, Otake Y, Moriguchi A, Ito T, Hirota Y, Kawamura K, et al. Iodine-123 metaiodobenzylguanidine myocardial scintigraphy for prediction of response to beta-blocker therapy in patients with dilated cardiomyopathy. Am Heart J 1997;133:353-8.
Acknowledgments
We would like to thank Julia Petryk and Richard Seymour for their technical assistance with the nuclear imaging, hemodynamic assessments, respectively.
Disclosures
RdK is a consultant for- and has received grant funding from Jubilant DraxImage. RdK receives revenues from Rubidium-82 generator technology licensed to Jubilant DraxImage, and from sales of FlowQuant software. RSB is or has been a consultant for- and has received grant funding from GE Healthcare, Lantheus Medical Imaging, and Jubilant DraxImage. The remaining authors have nothing to disclose.
Ethical approval
All animal received humane care and procedures conformed to the guiding principles of the National Institute of Health’s Guide for the Care and Use of Laboratory Animals, and were approved by the University of Ottawa Heart Institute Animal Care Committee.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarizes the contents of the paper and is free for re-use at meetings and presentations. Search for the article DOI on SpringerLink.com.
The authors have also provided an audio summary of the article, which is available to download as ESM, or to listen to via the JNC/ASNC Podcast.
Funding Jason Zelt is an MD/PhD student co-supervised by Drs Lisa Mielniczuk, Rob Beanlands and Rob deKemp and supported in part by the Vanier Canada Graduate Scholarship and The University of Ottawa. Rob Beanlands is a Career Investigator supported by the Heart and Stroke Foundation of Ontario (HSFO) and is a Tier 1 Chair in Cardiac Imaging Research at the University of Ottawa and the Vered Chair in Cardiology at the University of Ottawa Heart Institute. Lisa Mielniczuk is a Mid-career Clinician Scientist supported by HSFO and a Tier 2 Chair in HF Research at the University of Ottawa. This work was supported in part by a grant the Heart and Stroke Foundation of Canada (G-17-0018315).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zelt, J.G.E., Schock, S., deKemp, R.A. et al. [11C]meta-hydroxyephedrine PET evaluation in experimental pulmonary arterial hypertension: Effects of carvedilol of right ventricular sympathetic function. J. Nucl. Cardiol. 28, 407–422 (2021). https://doi.org/10.1007/s12350-020-02494-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-020-02494-6