Abstract
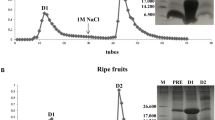
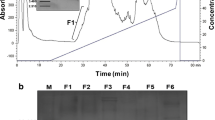
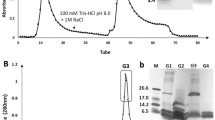
Antimicrobial peptides (AMPs) are molecules present in several life forms, possess broad-spectrum of inhibitory activity against pathogenic microorganisms, and are a promising alternative to combat the multidrug resistant pathogens. The aim of this work was to identify and characterize AMPs from Capsicum chinense fruits and to evaluate their inhibitory activities against yeasts of the genus Candida and α-amylases. Initially, after protein extraction from fruits, the extract was submitted to anion exchange chromatography resulting two fractions. Fraction D1 was further fractionated by molecular exclusion chromatography, and three fractions were obtained. These fractions showed low molecular mass peptides, and in fraction F3, only two protein bands of approximately 6.5 kDa were observed. Through mass spectrometry, we identified that the lowest molecular mass protein band of fraction F3 showed similarity with AMPs from plant defensin family. We named this peptide CcDef3 (Capsicum chinense defensin 3). The antifungal activity of these fractions was analyzed against yeasts of the genus Candida. At 200 μg/mL, fraction F1 inhibited the growth of C. tropicalis by 26%, fraction F2 inhibited 35% of the growth of C. buinensis, and fraction F3 inhibited all tested yeasts, exhibiting greater inhibition activity on the growth of the yeast C. albicans (86%) followed by C. buinensis (69%) and C. tropicalis (21%). Fractions F1 and F2 promoted membrane permeabilization of all tested yeasts and increased the endogenous induction of reactive oxygen species (ROS) in C. buinensis and C. tropicalis, respectively. We also observed that fraction F3 at a concentration of 50 µg/mL inhibited the α-amylase activities of Tenebrio molitor larvae by 96% and human salivary by 100%. Thus, our results show that fraction F3, which contains CcDef3, is a very promising protein fraction because it has antifungal potential and is able to inhibit the activity of different α-amylase enzymes.






Similar content being viewed by others
References
Pennisi E (2010) Armed and dangerous. Science 327(5967):804–805. https://doi.org/10.1126/science.327.5967.804
Parisi K, Shafee TMA, Quimbar P et al (2019) The evolution, function and mechanisms of action for plant defensins. Semin Cell Dev Biol 88:107–118. https://doi.org/10.1016/j.semcdb.2018.02.004
Bongomin F, Gago S, Oladele RO, Denning DW (2017) Global and multi-national prevalence of fungal diseases-estimate precision. J Fungi 3:57. https://doi.org/10.3390/jof3040057
Ullivarri MF, Arbulu S, Garcia-Gutierrez E, Cotter PD (2020) Antifungal peptides as therapeutic agents. Front Cell Infect Microbiol 10:105. https://doi.org/10.3389/fcimb.2020.00105
Oliveira JTA, Souza PFN, Vasconcelos IM et al (2019) Mo-CBP3-PepI, Mo-CBP3-PepII, and Mo-CBP3-PepIII are synthetic antimicrobial peptides active against human pathogens by stimulating ROS generation and increasing plasma membrane permeability. Biochimie 157:10–21. https://doi.org/10.1016/j.biochi.2018.10.016
Galdiero E, Lombardi L, Falanga A et al (2019) Biofilms: novel strategies based on antimicrobial peptides. Pharmaceutics 11(7):322. https://doi.org/10.3390/pharmaceutics11070322
Cools TL, Vriens K, Struyfs C et al (2017) The antifungal plant defensin HsAFP1 is a phosphatidic acid-interacting peptide inducing membrane permeabilization. Front Microbiol 8:2295. https://doi.org/10.3389/fmicb.2017.02295
Nicola AM, Albuquerque P, Paes HC et al (2019) Antifungal drugs: new insights in research e development. Pharmacol Ther 195:21–38. https://doi.org/10.1016/j.pharmthera.2018.10.008
Koehbach J, Craik DJ (2019) The vast structural diversity of antimicrobial peptides. Trends Pharmacol Sci 40:517–528. https://doi.org/10.1016/j.tips.2019.04.012
Patel S, Akhtar N (2017) Antimicrobial peptides (AMPs): the quintessential ‘offense and defense’ molecules are more than antimicrobials. Biomed Pharmacother 95:1276–1283. https://doi.org/10.1016/j.biopha.2017.09.042
da Cunha NB, Cobacho NB, Viana JFC et al (2017) The next generation of antimicrobial peptides (AMPs) as molecular therapeutic tools for the treatment of diseases with social and economic impacts. Drug Discov Today 22:234–248. https://doi.org/10.1016/j.drudis.2016.10.017
Watkins RR, Bonomo RA (2016) Overview: global and local impact of antibiotic resistance. Infect Dis Clin North Am 30:313–322. https://doi.org/10.1016/j.idc.2016.02.001
Andrade EKV, Rodrigues R, Bard GCV et al (2020) Identification, biochemical characterization and biological role of defense proteins from common bean genotypes seeds in response to Callosobruchus maculatus infestation. J Stored Prod Res 87:101580. https://doi.org/10.1016/j.jspr.2020.101580
Guillén-Chable F, Arenas-Sosa I, Islas-Flores I et al (2017) Antibacterial activity and phospholipid recognition of the recombinant defensin J1–1 from Capsicum genus. Protein Expr Purif 136:45–51. https://doi.org/10.1016/j.pep.2017.06.007
Pereira LS, Nascimento VV, Ribeiro SFF et al (2018) Characterization of Capsicum annuum L. leaf and root antimicrobial peptides: antimicrobial activity against phytopathogenic microorganisms. Acta Physiol Plant 40:107. https://doi.org/10.1007/s11738-018-2685-9
Taveira GB, Carvalho AO, Rodrigues R et al (2016) Thionin-like peptide from Capsicum annuum fruits: mechanism of action and synergism with fluconazole against Candida species. BMC Microbiol 16:12. https://doi.org/10.1186/s12866-016-0626-6
Maracahipes AC, Taveira GB, Sousa-Machado GBLY et al (2019) Characterization and antifungal activity of a plant peptide expressed in the interaction between Capsicum annuum fruits and the anthracnose fungus. Biosci Rep 39:12. https://doi.org/10.1042/BSR20192803
Afroz M, Akter S, Ahmed A et al (2020) Ethnobotany and antimicrobial peptides from plants of the Solanaceae family: an update and future prospects. Front Pharmacol 11:565. https://doi.org/10.3389/fphar.2020.00565
Taveira GB, Da Motta OV, Machado OLT et al (2014) Thionin-like peptides from Capsicum annuum fruits with high activity against human pathogenic bacteria and yeasts. Biopolym - Pept Sci Sect 102:30–39. https://doi.org/10.1002/bip.22351
Schägger H, von Jagow G (1987) Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem 166:368–379. https://doi.org/10.1016/0003-2697(87)90587-2
Shevchenko A, Tomas H, Havliš J et al (2007) In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat Protoc 1:2856–2860. https://doi.org/10.1038/nprot.2006.468
Carneiro RF, De Melo AA, De Almeida AS et al (2013) H-3, a new lectin from the marine sponge Haliclona caerulea: purification and mass spectrometric characterization. Int J Biochem Cell Biol 45:2864–2873. https://doi.org/10.1016/j.biocel.2013.10.005
Broekaert WF, Terras FRG, Cammue BPA, Vanderleyden J (1990) An automated quantitative assay for fungal growth inhibition. FEMS Microbiol Lett 69:55–59. https://doi.org/10.1016/0378-1097(90)90412-J
Thevissen K, Terras FRG, Broekaert WF (1999) Permeabilization of fungal membranes by plant defensins inhibits fungal growth. Appl Environ Microbiol 65:5451–5458. https://doi.org/10.1128/aem.65.12.5451-5458.1999
Mello EO, Ribeiro SFF, Carvalho AO et al (2011) Antifungal activity of PvD1 defensin involves plasma membrane permeabilization, inhibition of medium acidification, and induction of ROS in fungi cells. Curr Microbiol 62:1209–1217. https://doi.org/10.1007/s00284-010-9847-3
Silva FCV, Nascimento VV, Fernandes KV et al (2018) Recombinant production and α-amylase inhibitory activity of the lipid transfer protein from Vigna unguiculata (L. Walp.) seeds. Process Biochem 65:205–212. https://doi.org/10.1016/j.procbio.2017.10.018
Smith PK, Krohn RI, Hermanson GT et al (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85. https://doi.org/10.1016/0003-2697(85)90442-7
Campos ML, Souza CM, Oliveira KBS et al (2018) The role of antimicrobial peptides in plant immunity. J Exp Bot 69:4997–5011. https://doi.org/10.1093/jxb/ery294
Diz MS, Carvalho AO, Ribeiro SFF et al (2011) Characterisation, immunolocalisation and antifungal activity of a lipid transfer protein from chili pepper (Capsicum annuum) seeds with novel α-amylase inhibitory properties. Physiol Plant 142:233–246. https://doi.org/10.1111/j.1399-3054.2011.01464.x
Ribeiro SFF, Taveira GB, Carvalho AO et al (2012) Antifungal and other biological activities of two 2S albumin-homologous proteins against pathogenic fungi. Protein J 31:59–67. https://doi.org/10.1007/s10930-011-9375-4
Ribeiro SFF, Silva MS, Da Cunha M et al (2012) Capsicum annuum L. trypsin inhibitor as a template scaffold for new drug development against pathogenic yeast. Anton Leeuw Int J G 101(3):657–670. https://doi.org/10.1007/s10482-011-9683-x
Nascimento VV, Mello EO, Carvalho EOLP et al (2015) PvD1 defensin, a plant antimicrobial peptide with inhibitory activity against Leishmania amazonensis. Biosci Rep 35:5. https://doi.org/10.1042/BSR20150060
Bard GCV, Zottich U, Souza TAM et al (2016) Purification, biochemical characterization, and antimicrobial activity of a new lipid transfer protein from Coffea canephora seeds. Genet Mol Res 15:4. https://doi.org/10.4238/gmr15048859
Gebara RS, Taveira GB, Santos LA et al (2020) Identification and characterization of two defensins from Capsicum annuum fruits that exhibit antimicrobial activity. Probiotics Antimicrob Proteins 12(3):1253–1265. https://doi.org/10.1007/s12602-020-09647-6
Meyer B, Houlné G, Pozueta-Romero J et al (1996) Fruit-specific expression of a defensin-type gene family in bell pepper. Upregulation during ripening and upon wounding. Plant Physiol 112:615–622. https://doi.org/10.1104/pp.112.2.615
Seo HH, Park S, Park S et al (2014) Overexpression of a defensin enhances resistance to a fruit-specific anthracnose fungus in pepper. PLoS ONE 9:5. https://doi.org/10.1371/journal.pone.0097936
Dias GB, Gomes VM, Zottich UP et al (2013) Isolation, characterization and antifungal activity of proteinase inhibitors from Capsicum chinense Jacq. seeds. Protein J 32:15–26. https://doi.org/10.1007/s10930-012-9456-z
Corrêa JAF, Evangelista AG, de Nazareth T, M, Luciano FB, (2019) Fundamentals on the molecular mechanism of action of antimicrobial peptides. Materialia 8:100494. https://doi.org/10.1016/j.mtla.2019.100494
Cotabarren J, Lufrano D, Parisi MG, Obregón WD (2020) Biotechnological, biomedical, and agronomical applications of plant protease inhibitors with high stability: A systematic review. Plant Sci 292:110398. https://doi.org/10.1016/j.plantsci.2019.110398
Kosikowska P, Lesner A (2016) Antimicrobial peptides (AMPs) as drug candidates: a patent review (2003–2015). Expert Opin Ther Pat 26:689–702. https://doi.org/10.1080/13543776.2016.1176149
Silva MS, Ribeiro SFF, Taveira GB et al (2017) Application and bioactive properties of CaTI, a trypsin inhibitor from Capsicum annuum seeds: membrane permeabilization, oxidative stress and intracellular target in phytopathogenic fungi cells. J Sci Food Agric 97:3790–3801. https://doi.org/10.1002/jsfa.8243
Almeida LHO, Oliveira CFR, Rodrigues MS et al (2020) Adepamycin: design, synthesis and biological properties of a new peptide with antimicrobial properties. Arch Biochem Biophys 691:108487. https://doi.org/10.1016/j.abb.2020.108487
Wang K, Dang W, Xie J et al (2015) Antimicrobial peptide protonectin disturbs the membrane integrity and induces ROS production in yeast cells. Biochim Biophys Acta - Biomembr 1848:2365–2373. https://doi.org/10.1016/j.bbamem.2015.07.008
De Coninck B, Cammue BPA, Thevissen K (2013) Modes of antifungal action and in planta functions of plant defensins and defensin-like peptides. Fungal Biol Rev 26:109–120. https://doi.org/10.1016/j.fbr.2012.10.002
Soares JR, Melo EJT, da Cunha M et al (2017) Interaction between the plant ApDef1 defensin and Saccharomyces cerevisiae results in yeast death through a cell cycle- and caspase-dependent process occurring via uncontrolled oxidative stress. Biochim Biophys Acta - Gen Subj 1861:3429–3443. https://doi.org/10.1016/j.bbagen.2016.09.005
Mello EO, Taveira GB, Carvalho AO, Gomes VM (2019) Improved smallest peptides based on positive charge increase of the γ-core motif from PνD1 and their mechanism of action against Candida species. Int J Nanomedicine 14:407–420. https://doi.org/10.2147/IJN.S187957
Moore J, Rajasekaran K, Cary JW, Chlan C (2019) Mode of action of the antimicrobial peptide D4E1 on Aspergillus flavus. Int J Pept Res Ther 25:1135–1145. https://doi.org/10.1007/s10989-018-9762-1
Teixeira V, Feio MJ, Bastos M (2012) Role of lipids in the interaction of antimicrobial peptides with membranes. Prog Lipid Res 51:149–177. https://doi.org/10.1016/j.plipres.2011.12.005
Shackelford RE, Kaufmann WK, Paules RS (2000) Oxidative stress and cell cycle checkpoint function. Free Radic Biol Med 28:1387–1404. https://doi.org/10.1016/S0891-5849(00)00224-0
Santos LA, Taveira GB, Ribeiro SFF et al (2017) Purification and characterization of peptides from Capsicum annuum fruits which are α-amylase inhibitors and exhibit high antimicrobial activity against fungi of agronomic importance. Protein Expr Purif 132:97–107. https://doi.org/10.1016/j.pep.2017.01.013
Silva MS, Ribeiro SFF, Taveira GB et al (2018) Partial characterization of a immune-related lipid transfer protein (LTP) and activity against yeasts of medical interest from proteins of Canavalia ensiformes (Jack-bean) seeds. World J Pharm Res 7:1120–1138. https://doi.org/10.20959/wjpr201815-13076
Payan F (2004) Structural basis for the inhibition of mammalian and insect α-amylases by plant protein inhibitors. Biochim Biophys Acta - Proteins Proteomics 1696:171–180. https://doi.org/10.1016/j.bbapap.2003.10.012
Silva SM, Koehnlein EA, Bracht A et al (2014) Inhibition of salivary and pancreatic α-amylases by a pinhão coat (Araucaria angustifolia) extract rich in condensed tannin. Food Res Int 56:1–8. https://doi.org/10.1016/j.foodres.2013.12.004
Feng GH, Richardson M, Chen MS et al (1996) α-Amylase inhibitors from wheat: amino acid sequences and patterns of inhibition of insect and human α-amylases. Insect Biochem Mol Biol 26:419–426. https://doi.org/10.1016/0965-1748(95)00087-9
Acknowledgments
This work was performed at the Universidade Estadual do Norte Fluminense Darcy Ribeiro. We are grateful to Souza L.C.D. and Kokis V.M. for general laboratory technical support.
Funding
This study was financed by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001, Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) (E-26/203.090/2016; E-26/202.132/2015; E-26/202.330/2019), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aguieiras, M.C.L., Resende, L.M., Souza, T.A.M. et al. Potent Anti-Candida Fraction Isolated from Capsicum chinense Fruits Contains an Antimicrobial Peptide That is Similar to Plant Defensin and is Able to Inhibit the Activity of Different α-Amylase Enzymes. Probiotics & Antimicro. Prot. 13, 862–872 (2021). https://doi.org/10.1007/s12602-020-09739-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-020-09739-3