Abstract
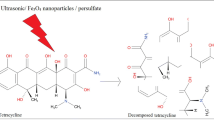
Nowadays, the existence of antibiotic compounds in pharmaceutical wastewaters is one of the new problems that can be considered in environmental pollution. The removal of ciprofloxacin from aqueous solutions is examined in the present study by using peroxymonosulfate (Oxone=PMS) activated with hybridized ultrasonic waves and synthesized ZnO nanoparticles. Operational parameters like ZnO nanoparticles dosage, initial pH, and peroxymonosulfate concentration, and their effect on the removal of the antibiotic have been investigated. The results showed 98.3% antibiotics removal observed with an initial pH of 3, ZnO nanoparticles dosage of 1 g/L, and peroxymonosulfate concentration of 1 g/L. This process had a high removal efficiency and was suitable for the removal of ciprofloxacin from aquatic environments.








Similar content being viewed by others
References
Wynnae O. Antibiotics in the environment TESC 422 case study paper. 2003.
Alijani, H. Q., Pourseyedi, S., Torkzadeh-Mahani, M., Seifalian, A., & Khatami, M. (2020). Bimetallic nickel-ferrite nanorod particles: Greener synthesis using rosemary and its biomedical efficiency. Artificial Cells, Nanomedicine, and Biotechnology., 48(1), 242–251.
Gagnon, C., Lajeunesse, A., Cejka, P., Gagne, F., & Hausler, R. (2008). Degradation of selected acidic and neutral pharmaceutical products in a primary-treated wastewater by disinfection processes. Ozone: Science and Engineering., 30(5), 387–392.
Dirany, A., Sirés, I., Oturan, N., & Oturan, M. A. (2010). Electrochemical abatement of the antibiotic sulfamethoxazole from water. Chemosphere., 81(5), 594–602.
Capriotti, A. L., Cavaliere, C., Piovesana, S., Samperi, R., & Laganà, A. (2012). Multiclass screening method based on solvent extraction and liquid chromatography–tandem mass spectrometry for the determination of antimicrobials and mycotoxins in egg. Journal of Chromatography A., 1268, 84–90.
Peng, H., Pan, B., Wu, M., Liu, Y., Zhang, D., & Xing, B. (2012). Adsorption of ofloxacin and norfloxacin on carbon nanotubes: Hydrophobicity-and structure-controlled process. Journal of hazardous materials., 233, 89–96.
Bushra, M. U., Huda, M. N., Mostafa, M., Sultan, M. Z., & Rahman, A. (2013). Study of forced degradation of ciprofloxacin HCl indicating stability using RP-HPLC method Der. Pharm Chem., 5, 132–137.
Ali, S. A., Mmuo, C. C., Abdulraheem, R. O., Abdulkareem, S. S., Alemika, E. T., Sani, M. A., et al. (2011). High performance liquid chromatography (HPLC) method development and validation indicating assay for ciprofloxacin hydrochloride. Journal of Applied Pharmaceutical Science., 1(8), 239.
Sun, S.-P., Guo, H.-Q., Ke, Q., Sun, J.-H., Shi, S.-H., Zhang, M.-L., et al. (2009). Degradation of antibiotic ciprofloxacin hydrochloride by photo-Fenton oxidation process. Environmental Engineering Science., 26(4), 753–759.
Wei, R., Ge, F., Huang, S., Chen, M., & Wang, R. (2011). Occurrence of veterinary antibiotics in animal wastewater and surface water around farms in Jiangsu Province. China. Chemosphere., 82(10), 1408–1414.
Le-Minh, N., Khan, S., Drewes, J., & Stuetz, R. (2010). Fate of antibiotics during municipal water recycling treatment processes. Water Research, 44(15), 4295–4323.
Khazeni, S., Hatamian-Zarmi, A., Yazdian, F., Mokhtari-Hosseini, Z. B., Ebrahimi-Hosseinzadeh, B., Noorani, B., et al. (2017). Production of nanocellulose in miniature-bioreactor: optimization and characterization. Preparative Biochemistry and Biotechnology., 47(4), 371–378.
Klavarioti, M., Mantzavinos, D., & Kassinos, D. (2009). Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes. Environment international., 35(2), 402–417.
Foong, L. K., Foroughi, M. M., Mirhosseini, A. F., Safaei, M., Jahani, S., Mostafavi, M., et al. (2020). Applications of nano-materials in diverse dentistry regimes. RSC Advances., 10(26), 15430–15460.
Lin, A. Y.-C., Lin, C.-F., Chiou, J.-M., & Hong, P. A. (2009). O3 and O3/H2O2 treatment of sulfonamide and macrolide antibiotics in wastewater. Journal of hazardous materials., 171(1–3), 452–458.
Koyuncu, I., Arikan, O. A., Wiesner, M. R., & Rice, C. (2008). Removal of hormones and antibiotics by nanofiltration membranes. Journal of membrane science., 309(1–2), 94–101.
Kim, T.-H., Kim, S. D., Kim, H. Y., Lim, S. J., Lee, M., & Yu, S. (2012). Degradation and toxicity assessment of sulfamethoxazole and chlortetracycline using electron beam, ozone and UV. Journal of hazardous materials., 227, 237–242.
Choi, K.-J., Son, H.-J., & Kim, S.-H. (2007). Ionic treatment for removal of sulfonamide and tetracycline classes of antibiotic. Science of the total environment., 387(1–3), 247–256.
Choi, K.-J., Kim, S.-G., & Kim, S.-H. (2008). Removal of antibiotics by coagulation and granular activated carbon filtration. Journal of hazardous materials., 151(1), 38–43.
Elmolla, E. S., & Chaudhuri, M. (2010). Degradation of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution by the UV/ZnO photocatalytic process. Journal of hazardous materials., 173(1–3), 445–449.
Rauf, M., & Ashraf, S. S. (2009). Fundamental principles and application of heterogeneous photocatalytic degradation of dyes in solution. Chemical engineering journal., 151(1–3), 10–18.
Intarasuwan, K., Amornpitoksuk, P., Suwanboon, S., & Graidist, P. (2017). Photocatalytic dye degradation by ZnO nanoparticles prepared from X2C2O4 (X= H, Na and NH4) and the cytotoxicity of the treated dye solutions. Separation and Purification Technology., 177, 304–312.
Pan, L., Muhammad, T., Ma, L., Huang, Z.-F., Wang, S., Wang, L., et al. (2016). MOF-derived C-doped ZnO prepared via a two-step calcination for efficient photocatalysis. Applied Catalysis B: Environmental., 189, 181–191.
Daneshvar, N., Aber, S., Dorraji, M. S., Khataee, A., & Rasoulifard, M. (2007). Photocatalytic degradation of the insecticide diazinon in the presence of prepared nanocrystalline ZnO powders under irradiation of UV-C light. Separation and purification Technology., 58(1), 91–98.
Alkasir, M., Samadi, N., Sabouri, Z., Mardani, Z., Khatami, M., & Darroudi, M. (2020). Evaluation cytotoxicity effects of biosynthesized zinc oxide nanoparticles using aqueous Linum Usitatissimum extract and investigation of their photocatalytic activity. Inorganic Chemistry Communications., 108066.
Segura, Y., Martínez, F., Melero, J. A., Molina, R., Chand, R., & Bremner, D. H. (2012). Enhancement of the advanced Fenton process (Fe0/H2O2) by ultrasound for the mineralization of phenol. Applied Catalysis B: Environmental., 113, 100–106.
Eskandarloo, H., Badiei, A., Behnajady, M. A., & Ziarani, G. M. (2016). Ultrasonic-assisted degradation of phenazopyridine with a combination of Sm-doped ZnO nanoparticles and inorganic oxidants. Ultrasonics sonochemistry., 28, 169–177.
Soltani, R. D. C., Jorfi, S., Ramezani, H., & Purfadakari, S. (2016). Ultrasonically induced ZnO–biosilica nanocomposite for degradation of a textile dye in aqueous phase. Ultrasonics sonochemistry., 28, 69–78.
Ahmed, M. M., Barbati, S., Doumenq, P., & Chiron, S. (2012). Sulfate radical anion oxidation of diclofenac and sulfamethoxazole for water decontamination. Chemical Engineering Journal., 197, 440–447.
Wei, C., Zhang, J., Zhang, Y., Zhang, G., Zhou, P., Li, W., et al. (2017). Ultrasound enhanced heterogeneous activation of peroxymonosulfate by a co-NiOx catalyst. Water Science and Technology., 76(6), 1436–1446.
Ahmadi, S., Banach, A., Mostafapour, F. K., & Balarak, D. (2017). Study survey of cupric oxide nanoparticles in removal efficiency of ciprofloxacin antibiotic from aqueous solution: Adsorption isotherm study. Desalination and water treatment., 89, 297–303.
Cai, C., Zhang, H., Zhong, X., & Hou, L. (2014). Electrochemical enhanced heterogeneous activation of peroxydisulfate by Fe–co/SBA-15 catalyst for the degradation of Orange II in water. Water Research, 66, 473–485.
Hou, L., Zhang, H., Wang, L., Chen, L., Xiong, Y., & Xue, X. (2013). Removal of sulfamethoxazole from aqueous solution by sono-ozonation in the presence of a magnetic catalyst. Separation and Purification Technology., 117, 46–52.
Zhang, H., Zhang, J., Zhang, C., Liu, F., & Zhang, D. (2009). Degradation of CI acid Orange 7 by the advanced Fenton process in combination with ultrasonic irradiation. Ultrasonics sonochemistry., 16(3), 325–330.
Wang, X., Wang, L., Li, J., Qiu, J., Cai, C., & Zhang, H. (2014). Degradation of acid Orange 7 by persulfate activated with zero valent iron in the presence of ultrasonic irradiation. Separation and Purification Technology., 122, 41–46.
Rahdar, S., & Ahmadi, S. (2019). The removal of amoxicillin with Zno nanoparticles in combination with US-H2O2 advanced oxidation processes from aqueous solutions. Iranian Journal of Health Sciences., 7(1), 36–45.
Liu, F., Yi, P., Wang, X., Gao, H., & Zhang, H. (2018). Degradation of acid Orange 7 by an ultrasound/ZnO-GAC/persulfate process. Separation and Purification Technology., 194, 181–187.
Kamani, H., Bazrafshan, E., Ashrafi, S. D., & Sancholi, F. (2017). Efficiency of sono-nano-catalytic process of TiO2 nano-particle in removal of erythromycin and metronidazole from aqueous solution. Journal of Mazandaran University of Medical Sciences., 27(151), 140–154.
Hao, F., Guo, W., Wang, A., Leng, Y., & Li, H. (2014). Intensification of sonochemical degradation of ammonium perfluorooctanoate by persulfate oxidant. Ultrasonics sonochemistry., 21(2), 554–558.
Liang, C., Wang, Z.-S., & Bruell, C. J. (2007). Influence of pH on persulfate oxidation of TCE at ambient temperatures. Chemosphere., 66(1), 106–113.
Sathishkumar, P., Pugazhenthiran, N., Mangalaraja, R. V., Asiri, A. M., & Anandan, S. (2013). ZnO supported CoFe2O4 nanophotocatalysts for the mineralization of direct blue 71 in aqueous environments. Journal of hazardous materials., 252, 171–179.
Zhang, X., Zhang, J., Huang, X., Wu, Q. P., Yan, C. H., & Lu, J. F. (2019). Efficient PMS activation by Zn/Fe-MOF s-derived ZnO/Fe3O4@ carbon spheres for the degradation of acid Orange 7. Water Environment Research.
Molinari, R., Pirillo, F., Loddo, V., & Palmisano, L. (2006). Heterogeneous photocatalytic degradation of pharmaceuticals in water by using polycrystalline TiO2 and a nanofiltration membrane reactor. Catalysis Today., 118(1–2), 205–213.
Gad-Allah, T. A., Ali, M. E., & Badawy, M. I. (2011). Photocatalytic oxidation of ciprofloxacin under simulated sunlight. Journal of hazardous materials., 186(1), 751–755.
Salari, M., Rakhshandehroo, G. R., & Nikoo, M. R. (2018). Degradation of ciprofloxacin antibiotic by homogeneous Fenton oxidation: Hybrid AHP-PROMETHEE method, optimization, biodegradability improvement and identification of oxidized by-products. Chemosphere., 206, 157–167.
Khoshnamvand, N., Mostafapour, F. K., Mohammadi, A., & Faraji, M. (2018). Response surface methodology (RSM) modeling to improve removal of ciprofloxacin from aqueous solutions in photocatalytic process using copper oxide nanoparticles (CuO/UV). AMB Express, 8(1), 48.
Zhang, Y., Tran, H. P., Du, X., Hussain, I., Huang, S., Zhou, S., et al. (2017). Efficient pyrite activating persulfate process for degradation of p-chloroaniline in aqueous systems: A mechanistic study. Chemical Engineering Journal., 308, 1112–1119.
Wang, A., Zhang, Y., Zhong, H., Chen, Y., Tian, X., Li, D., et al. (2018). Efficient mineralization of antibiotic ciprofloxacin in acid aqueous medium by a novel photoelectro-Fenton process using a microwave discharge electrodeless lamp irradiation. Journal of hazardous materials., 342, 364–374.
Vasconcelos, T. G., Henriques, D. M., König, A., Martins, A. F., & Kümmerer, K. (2009). Photo-degradation of the antimicrobial ciprofloxacin at high pH: Identification and biodegradability assessment of the primary by-products. Chemosphere., 76(4), 487–493.
Zhang, C.-L., Qiao, G.-L., Zhao, F., & Wang, Y. (2011). Thermodynamic and kinetic parameters of ciprofloxacin adsorption onto modified coal fly ash from aqueous solution. Journal of Molecular Liquids., 163(1), 53–56.
Nekouei, F., & Nekouei, S. (2017). Comparative study of photocatalytic activities of Zn5 (OH) 8Cl2· H2O and ZnO nanostructures in ciprofloxacin degradation: Response surface methodology and kinetic studies. Science of the Total Environment., 601, 508–517.
Nasiri, A., Tamaddon, F., Mosslemin, M. H., Amiri Gharaghani, M., & Asadipour, A. (2019). Magnetic nano-biocomposite CuFe2O4@ methylcellulose (MC) prepared as a new nano-photocatalyst for degradation of ciprofloxacin from aqueous solution. Environmental Health Engineering and Management Journal., 6(1), 41–51.
Mondal, S. K., Saha, A. K., & Sinha, A. (2018). Removal of ciprofloxacin using modified advanced oxidation processes: Kinetics, pathways and process optimization. Journal of cleaner production., 171, 1203–1214.
Peng, G., Li, T., Ai, B., Yang, S., Fu, J., He, Q., et al. (2019). Highly efficient removal of enrofloxacin by magnetic montmorillonite via adsorption and persulfate oxidation. Chemical Engineering Journal., 360, 1119–1127.
Funding
None.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Research Involving Humans and Animals Statement
None.
Informed Consent
None.
Conflict of Interest
None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Firouzeh, N., Malakootian, M., Asadzadeh, S.N. et al. Degradation of Ciprofloxacin Using Ultrasound/ZnO/Oxone Process from Aqueous Solution-Lab-Scale Analysis and Optimization. BioNanoSci. 11, 306–313 (2021). https://doi.org/10.1007/s12668-021-00838-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-021-00838-1