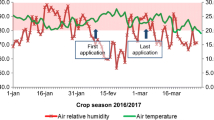
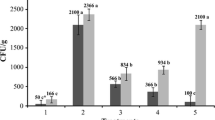
Abstract
Since it was first reported in Brazil, Asian soybean rust has been considered the most significant disease in the crop. Successive applications of fungicides during the crop cycle have been the most efficient control measures. Considering the occurrence of Phakopsora pachyrhizi populations with less sensitivity to the main recommended fungicide molecules, which results in lower control efficiency, as well as difficulties in obtaining new molecules, it is important that new tools be tested for integration into a management program. In field conditions, the efficiencies of one biological product based on Bacillus subtilis and another based on roasted coffee bean oils were studied in sequential and alternating applications with the fungicide pyraclostrobin + epoxiconazole. The application schedules of B. subtilis and chemical fungicide products in both trials reduced the area under the disease progress curve and increased the yield, the weight of 100 seeds, and the normalized difference vegetation index when compared to the control. B. subtilis and fungicides applied in sequence reduced the area under the disease progress curve 41% to 53% and 67% to 69% in the first and second assays, respectively. The applications of coffee oil alone or alternate with fungicide did not increased productivity compared to the control in the two fields. These results were obtained under conditions with a low intensity of disease, and therefore, different responses may be found under high intensity levels. These results allow us to suggest the use of products based on B. subtilis in soybean rust management programs along with chemical fungicides under low-medium disease pressure.

Similar content being viewed by others
References
Agrofit (2017) Sistema de agrotóxicos fitossanitários do Ministério da Agricultura, Pecuária e Abastecimento. http://extranet.agricultura.gov.br/agrofit_cons/principal_agrofit_cons. Acessed 10 Feb 2017
Agrofit (2019) Sistema de agrotóxicos fitossanitários do Ministério da Agricultura, Pecuária e Abastecimento http://extranetagriculturagovbr/agrofit_cons/principal_agrofit_cons Acessed 7 Nov 2019
Avozani A, Reis EM, Tonin RB (2014) Sensitivity loss by Corynespora cassiicola, isolated from soybean, to the fungicide carbendazim. Summa Phytopathol 40:273–276
Baker CJ, Stavely JR, Mock N (1985) Biocontrol of bean rust by Bacillus subtilis under field conditions. Plant Dis 69:770–772
Barbosa MZ, Sampaio RM (2017) Soja: alta produtividade e tecnologia. Análises e Indicadores do Agronegócio 12(5):1–5
Bettiol W, Várzea VMP (1992) Controle biológico da ferrugem (Hemileia vastatrix) do cafeeiro com Bacillus subtilis em condições controladas. Fitopatol Bras 17:91–95
Bettiol W, Brandão MSB, Saito ML (1992) Controle da ferrugem do feijoeiro com extratos e células formuladas de Bacillus subtilis. Summa Phytopathol 18:153–159
Bettiol W, Saito ML, Brandão MSB (1994) Controle da ferrugem do cafeeiro com produtos à base de Bacillus subtilis. Summa Phytopathol 20:119–123
Brum RBCS, Castro GC, Gama FR, Cardon CH, Santos GR (2014) Phytotoxicity of essential oils in watermelon, bean and rice plants. J Biotec Biodivers 5:101–109
Cabral WC, Diniz Campos H, Costa LSAS, Simon A (2016) In vitro susceptibility of Corynespora cassiicola isolate from Brazil fields to fungicide. Afric J Agric Res 11:1699–1711
Cawoy H, Bettiol W, Fickers P, Ongena M (2011) Bacillus-based biological control of plant diseases. In: Stoytcheva M (ed) Pesticides in the modern world – pesticides use and management. Rijeka, Croatia, InTech, pp 274–302
Cawoy H, Debois D, Franzil L, Pauw ED, Thonart P, Ongena M (2014) Lipopeptides as main ingredients for inhibition of fungal phytopathogens by Bacillus subtilis/amyloliquefaciens. Microb Biotech 8:281–295
Chen WY, Ko WH (2015) Flaxseed oil for control of peanut rust and its disease control mechanism. Phytopathology 163:271–278
CONAB (2018) Companhia Nacional de Abastecimento. Acompanhamento da safra brasileira: grãos: safra 2017/2018. v. 4, Publicação Online. http://www.conab.gov.br/OlalaCMS/uploads/arquivos/18_01_11_14_17_49_graos_4o_levantamento.pdf. Accessed in 29 Jan 2018
Dorighello DV, Bettiol W, Maia NB, Leite RMVBC (2015) Controlling Asian soybean rust (Phakopsora pachyrhizi) with Bacillus spp. and coffee oil. Crop Prot 67:59–65
Edgecomb DW, Manker DC (2008) Serenade (Bacillus subtilis strain QST713) and sonata (Bacillus pumilus strain QST2808), new biological tools for integrated and organic disease control programs. Summa Phytopathol 34S:196–199
Fontal EML (2007) Extraccion de aceite de cafe. Rev Ing Investig 27:25–31
Fousia S, Paplomatas EJ, Tjamos S (2016) Bacillus subtilis QST 713 confers protection to tomato plants against Pseudomonas syringae pv tomato and induces plant defence-related genes. J Phytopathol 164:264–270
FRAC (2010) Fungicide resistance action committee. Recommendations for fungicide mixtures designed to delay resistance evolution. http://www.frac.info/docs/default-source/publications/frac-recommendations-for-fungicide-mixtures/frac-recommendations-for-fungicide-mixtures%2D%2D-january-2010.pdf?sfvrsn=4. Accessed on 15 Oct 2016
Gao X, Han Q, Chen Y, Qin H, Huang L, Kang Z (2014) Biological control of oilseed rape Sclerotinia stem rot by Bacillus subtilis strain Em7. Biocontrol Sci Tech 24:39–52
Godoy CV, Koga LJ, Canteri MG (2006) Diagrammatic scale for assessment of soybean rust severity. Fitopatol Bras 31:63–68
Godoy CV, Seixas CDS, Soares RM, Marcelino-Guimarães FC, Meyer MC, Costamilan LM (2016) Asian soybean rust in Brazil: past, present, and future. Pesq agropec bras 51:407–421
Goellner K, Loehrer M, Langenbach C, Uwe Conrath U, Kock E, Schaffrath U (2010) Phakopsora pachyrhizi, the causal agent of Asian soybean rust. Mol Plant Pathol 11:169–177
Goulart ACP, Furlan SH, Fujino MT (2011) Controle integrado da ferrugem asiática da soja (Phakopsora pachyrhizi) com o fungicida fluquinconazole aplicado nas sementes em associação com outros fungicidas pulverizados na parte aérea da cultura. Summa Phytopathol 37:113–118
Guan Q, Huang W, Zhao J, Liu L, Liang D, Huang L, Wang L, Yang G (2014) Quantitative indemnification of yellow rust, powdery mildew and fertilizer water stress in winter wheat using in-situ hyperspectral data. Sens Lett 12:876–882
Haddad F, Maffia LA, Mizubuti ESG, Teixeira H (2009) Biological control of coffee rust by antagonistic bacteria under field conditions in Brazil. Biol Control 49:114–119
Hui L, Jhao J, Feng H, Huang L, Kang Z (2013) Biocontrol of wheat stripe rust by an endophytic Bacillus subtilis strain E1R-j in green house and field trials. Crop Prot 43:201–206
Jahagirdar S, Patil PV, Benagi VI (2012) Bio-formulations and indigenous technology methods in the management of Asian soybean rust. Int J Plant Prot 5:63–67
Kawashima CG, Guimarães GA, Nogueira SR, MacLean D, Cook DR, Steuernagel B, Baek J, Bouyioukos C, Melo Bdo V, Tristão G, de Oliveira JC, Rauscher G, Mittal S, Panichelli L, Bacto K, Johnson E, Iyer G, Tabor G, Wuff BB, Ward E, Rairdan GJ, Broglie KE, Wu G, van Esse HP, Jones JD, Brommonschenkel SH (2016) A pigeonpea gene confers resistance to Asian soybean rust in soybean. Nat Biotechnol 34:661–665
Kawuki RS, Adipala E, Tukamuhabwa P (2003) Yield loss associated with soya bean rust (Phakopsora pachyrhizi Syd.) in Uganda. J Phytopathol 151:7–12
Kinsella K, Schulthess CP, Morris TM, Stuart JD (2009) Rapid quantification of Bacillus antibiotics in the rhizosphere. Soil Biol Biochem 41:374–379
Klosowski AC, May De Mio LL, Miessner S, Rodrigues R, Stammler G (2016) Detection of the F129L mutation in the cytochrome b gene in Phakopsora pachyrhizi. Pest Manag Sci 72:1211–1215
Langenbach C, Campe R, Beyer SF, Muller AN, Conrath U (2016) Fighting Asian soybean rust. Front Plant Sci 7:797
Li H, Zhao J, Feng H, Huang L, Kang Z, Li H (2013) Biological control of wheat stripe rust by an endophytic Bacillus subtilis strain E1R-j in greenhouse and field trials. Crop Prot 43:201–206
Liu M, Li S, Swaminathan S, Sahu BB, Leandro FL, Cardinal AJ, Bhattacharyya M, Song Q, Walker DR, Cianzio SR (2016) Identification of a soybean rust resistance gene in PI 567104B. Theor Appl Genet 129:863–877
Madden LV, Hughes G, van den Boschi F (2007) The study of plant disease epidemics. The American Phytopathological society, St. Paul, p 432
Mesquini RM, Schwan-Estrada KRF, Vieira RA, Nascimento JF (2011) Controle e progresso temporal da ferrugem asiática da soja sob controle alternativo em campo. Summa Phytopathol 37:24–29
Mohamed A, Hamza A, Berbalah A (2016) Recent approaches for controlling downy mildew of cucumber under greenhouse conditions. Plant Prot Sci 52:1–9
Murithi HM, Beed F, Tukamuhabwa P, Thomma BPHJ, Joosten MHAJ (2016) Soybean production in eastern and southern Africa and threat of yield loss due to soybean rust caused by Phakopsora pachyrhizi. Plant Pathol 65:176–188
Nagórska K, Bikowski M, Obuchowski M (2007) Multicellular behaviour and production of a wide variety of toxic substances support usage of Bacillus subtilis as a powerful biocontrol agent. Acta Biochim Pol 54:495–508
Neumaier N, Nepomuceno AL, Farias JRB, Oya T (2000) Estádios de desenvolvimento da cultura de soja. In: Bonato ER (ed) Estresses em soja. Passo Fundo, Embrapa Trigo, pp 19–44
Nunes CDM (2015) Controle de ferrugem asiática da soja baseado no número de aplicações de fungicida em diferentes estádios realizado em duas safras 2011/2012 e 2012/2013. In: Vernetti Junior FJ (ed) Resultados de pesquisa de soja na Embrapa Clima Temperado. Pelotas, Embrapa Clima Temperado (Documentos), pp 38–46
Olanrewaju OW, Glick BR, Babalola OO (2017) Mechanisms of action of plant growth promoting bacteria. World J Microb Biot 33:197
Ongena M, Jacques P (2008) Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol 16:115–125
Ongena M, Duby F, Jourdan E, Beaudry T, Jadin V, Dommes J, Thonart P (2005) Bacillus subtilis M4 decreases plant susceptibility towards fungal pathogens by increasing host resistance associated with differential gene expression. Appl Microbiol Biot 67:692–698
Paul C, Hartman GL, Wright DL, Walker DR (2013) First report of Phakopsora pachyrhizi adapting to soybean genotypes with Rpp1 or Rpp6 rust resistance genes in field plots in the United States. Plant Dis 97:1379
Peng D, Li S, Wang J, Chen C, Zhou M (2014a) Integrated biological and chemical control of rice sheath blight by Bacillus subtilis NJ-18 and jinggangmycin. Pest Manag Sci 70:258–263
Peng G, Lahlali R, Hwang S, Pageau D, Hynes RK, McDonald MR, Gossen BD, Strelkov SE (2014b) Crop rotation, cultivar resistance, and fungicides/biofungicides for managing clubroot (Plasmodiophora brassicae) on canola. Can J Plant Pathol 36:99–112
Reiss A, Jørgensen LN (2017) Biological control of yellow rust of wheat (Puccinia striiformis) with serenade® ASO (Bacillus subtilis strain QST713). Crop Prot 93:1–8
Ritchie SW, Hanway JJ, Thompson HE, Benson GO (1985) How a soybean plant develops. Iowa State University of Science and Technology, Ames, 20p
Saksirirat W, Hoppe HH (1991) Degradation of uredospores of the soybean rust fungus (Phakopsora pachyrhizi Syd.) by cell-free culture filtrates of the mycoparasite Verticillium psalliotae. J Phytopathol 132:33–45
Santiago R, Huiliñir C, Cottet L, Castillo A (2016) Microbiological characterization for a new wild strain of Paenibacillus polymyxa with antifungal activity against Botrytis cinerea. Biol Control 103:251–260
Schmitz HK, Medeiros CA, Craig IR, Stammler G (2014) Sensitivity of Phakopsora pachyrhizi towards quinone-outside inhibitors and demethylation-inhibitors, and corresponding resistance mechanisms. Pest Manag Sci 70:378–388
Shaner G, Finney RE (1977) The effect of nitrogen fertilization on the expression of slow-mildewing resistance in Knox wheat. Phytopathology 67:1051–1056
Silva AC, Souza PE, Amaral DC, Zeviani WM, Pinto JEBP (2014) Essential oils from Hyptis marrubioides, Aloysia gratíssima and Cordia verbenacea reduce the progress of Asian soybean rust. Acta Sci Agron 36:159–166
Simões K, Hawlik A, Rehfus A, Gava F, Stammler G (2018) First detection of a SDH variant with reduced SDHI sensitivity in Phakopsora pachyrhizi. J Plant Dis Protect 125:21–26
Standish JR, Tomaso-Peterson M, Allen TW, Sabanadzovic S (2015) Occurrence of QoI fungicide resistance in Cercospora sojina from Mississippi soybean. Plant Dis 99:1347–1352
Turatto MF, Dourado FS, Zilli JE, Botelho GR (2018) Control potential of Meloidogyne javanica and Ditylenchus spp. using fluorescent Pseudomonas and Bacillus spp. Braz J Microbiol 49:54–58
Twizeyimana M, Hartman GL (2017) Sensitivity of Phakopsora pachyrhizi isolates to fungicides and reduction infection based on fungicide and timing of application. Plant Dis 101:121–128
van Lenteren JC, Bolckmans K, Kohl J, Ravensberg WJ, Urbaneja A (2017) Biological control using invertebrates and microorganisms: plenty of new opportunities. BioControl 63:39–59
Ward NA, Robertson CL, Chanda AK, Schneide RW (2012) Effects of Simplicillium lanosoniveum on Phakopsora pachyrhizi, the soybean rust pathogen, and its use as a biological control agent. Phytopathology 102:749–760
Wei F, Hu X, Xu X (2016) Dispersal of Bacillus subtilis and its effect on strawberry phyllosphere microbiota under open field and protection conditions. Sci Rep 6:22611
Wu K, Su L, Fang Z, Yuan S, Wang L, Shen B, Shen Q (2017) Competitive use of root exudates by Bacillus amyloliquefaciens with Ralstonia solanacearum decreases the pathogenic population density and effectively controls tomato bacterial wilt. Sci Hortic 218:132–138
Xavier SA, Canteri MG, Barros DCM, Godoy CV (2013) Sensitivity of Corynespora cassiicola from soybean to carbendazim and prothioconazole. Trop Plant Pathol 38:431–435
Xu Z, Zhang R, Wang D, Qiu M, Feng H, Zhang N, Shen Q (2014) Enhanced control of cucumber wilt disease by Bacillus amyloliquefaciens SQR9 by altering the regulation of its DegU phosphorylation. Appl Environ Microbiol 80:2941–2950
Yuen GY, Steadman JR, Lindren DT, Schaff D, Jochum C (2001) Bean rust biological control using bacterial agents. Crop Prot 20:395–402
Zhang G, Pedersen KD, Phillips DV, Bradley CA (2012) Sensitivity of Cercospora sojina isolates to quinone outside inhibitor fungicides. Crop Prot 40:63–69
Acknowledgments
The authors are grateful to Fábio Brandi (Bayer Crop Science) and Nilson Molina Maia (IAC) for the donation of B. subtilis-based product and roasted coffee oil, respectively. The authors are grateful to the technicians of Embrapa, especially, Allan Misael Flausino and Ângelo Monico.
Funding
Dalton V. Dorighello acknowledges Coordenação de Aperfeiçoamento de Pessoal de Nível Superior for the scholarship and Wagner Bettiol acknowledges Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (305818/2015-5) for his productivity fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dorighello, D.V., Forner, C., de Campos Leite, R.M.V.B. et al. Management of Asian soybean rust with Bacillus subtilis in sequential and alternating fungicide applications. Australasian Plant Pathol. 49, 79–86 (2020). https://doi.org/10.1007/s13313-019-00677-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13313-019-00677-5