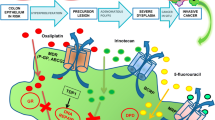
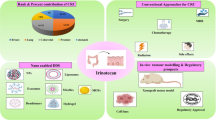
Abstract
Worldwide, colon cancer (CC) represents the fourth most common type of cancer and the fifth major cause of cancer-associated deaths. Surgical resection is considered the standard therapeutic choice for CC in early stages. However, in latter stages of the disease, adjuvant chemotherapy is essential for an appropriate management of this pathology. Metal-based complexes displaying cytotoxic properties towards tumor cells emerge as potential chemotherapeutic options. One metallodrug, oxaliplatin, was already approved for clinical use, playing an important role in the treatment of CC patients. Unfortunately, most of the newly designed metal-based complexes exhibit lack of selectivity against cancer cells, low solubility and permeability, high dose-limiting toxicity, and emergence of resistances. Nanodelivery systems enable the incorporation of metallodrugs at adequate payloads, solving the above-referred drawbacks. Moreover, drug delivery systems, depending on their physicochemical properties, are able to release the incorporated material preferentially at affected tissues/organs, enhancing the therapeutic activity in vivo, with concomitant fewer side effects. In this review, the general features and therapeutic management of CC will be addressed, with a special focus on preclinical or clinical studies using metal-based compounds. Furthermore, the use of different nanodelivery systems will also be described as tools to potentiate the therapeutic index of metallodrugs for the management of CC.
Graphical abstract













Similar content being viewed by others
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Nabi K, Le A. The intratumoral heterogeneity of cancer metabolism. Adv Exp Med Biol. 2018;1063:131–45.
Mattiuzzi C, Lippi G. Current Cancer Epidemiology glossary. J Epidemiol Glob Health. 2019;9:217–22.
Li F, Lai M. Colorectal cancer, one entity or three. J Zhejiang Univ Sci B. 2009;10:219–29.
Cappell MS. Pathophysiology, clinical presentation, and management of colon cancer. Gastroenterol Clin North Am. 2008;37:1–24.
Mattiuzzi C, Sanchis-Gomar F, Lippi G. Concise update on colorectal cancer epidemiology. Ann Transl Med. 2019;7:609.
Ahmed M. Colon cancer: a clinician’s perspective in 2019. Gastroenterol Res. 2020;13:1–10.
Ilyas M, Straub J, Tomlinson IPM, Bodmer WF. Genetic pathways in colorectal and other cancers. Eur J Cancer. 1999;35:1986–2002.
Balchen V, Simon K. Colorectal cancer development and advances in screening. Clin Interv Aging. 2016;11:967–76.
Virk GS, Jafri M, Mehdi S, Ashley C. Staging and survival of colorectal cancer (CRC) in octogenarians: Nationwide Study of US Veterans. J Gastrointest Oncol. 2018;10:12–8.
Johns Hopkins Colon Cancer Center. Sporadic (Nonhereditary) Colorectal Cancer: Introduction [Internet]. 2013 [cited 2020 Aug 4]. Available from: https://www.hopkinsmedicine.org/gastroenterology_hepatology/_pdfs/small_large_intestine/sporadic_nonhereditary_colorectal_cancer.pdf.
American Cancer Society. Survival Rates for Colorectal Cancer [Internet]. 2020 [cited 2020 Aug 26]. Available from: https://www.cancer.org/cancer/colon-rectal-cancer/detection-diagnosis-staging/survival-rates.html.
Pan P, Yu J, Wang LS. Colon cancer: what we eat. Surg Oncol Clin N Am. 2018;27:243–67.
Testino G, Leone S, Sumberaz A, Borro P. Alcohol and cancer. Alcohol Clin Exp Res. 2015;39:2261–2261.
Parajuli R, Bjerkaas E, Tverdal A, Selmer R, Le ML, Weiderpass E, et al. The increased risk of colon cancer due to cigarette smoking may be greater in women than men. Cancer Epidemiol Biomarkers Prev. 2013;22:862–71.
Baena R, Salinas P. Diet and colorectal cancer. Maturitas. 2015;80:258–64.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
GLOBOCAN [Internet]. 2020 [cited 2020 Aug 31]. Available from: https://gco.iarc.fr/.
Stoffel EM, Murphy CC. Epidemiology and mechanisms of the increasing incidence of colon and rectal cancers in young adults. Gastroenterology. 2020;158:341–53.
Schreuders EH, Ruco A, Rabeneck L, Schoen RE, Sung JJY, Young GP, et al. Colorectal cancer screening: a global overview of existing programmes. Gut. 2015;64:1637–49.
Burt RW. Colon cancer screening continues as pivotal to cancer prevention. J Natl Compr Cancer Netw. 2013;11:1457–8.
Vogel JD, Eskicioglu C, Weiser MR, Feingold DL, Steele SR. The American Society of Colon and Rectal Surgeons clinical practice guidelines for the treatment of colon cancer. Dis Colon Rectum. 2017;60:999–1017.
Labianca R, Beretta GD, Kildani B, Milesi L, Merlin F, Mosconi S, et al. Colon cancer. Crit Rev Oncol Hematol. 2010;74:106–33.
Hagan S, Orr MCM, Doyle B. Targeted therapies in colorectal cancer—an integrative view by PPPM. EPMA J. 2013;4:3.
Gulbake A, Jain A, Jain A, Jain A, Jain SK. Insight to drug delivery aspects for colorectal cancer. World J Gastroenterol. 2016;22:582–99.
National Cancer Institute. Colon Cancer Treatment (PDQ®): Health Professional Version. [Internet]. Bethesda (MD); 2020 [cited 2020 Aug 10]. Available from: https://www.cancer.gov/types/colorectal/hp/colon-treatment-pdq.
Hodgkinson N, Kruger CA, Abrahamse H. Targeted photodynamic therapy as potential treatment modality for the eradication of colon cancer and colon cancer stem cells. Tumor Biol. 2017;39:1010428317734691.
Willett CG, Duda DG, Czito BG, Bendell JC, Clark JW, Jain RK. Targeted therapy in rectal cancer. Oncology. 2007;21:1055–65.
Ashburn JH, Kalady MF. Radiation-induced problems in colorectal surgery. Clin Colon Rectal Surg. 2016;29:85–91.
Bender U, Rho YS, Barrera I, Aghajanyan S, Acoba J, Kavan P. Adjuvant therapy for stages II and III colon cancer: risk stratification, treatment duration, and future directions. Curr Oncol. 2019;26:S43-52.
Gelibter AJ, Caponnetto S, Urbano F, Emiliani A, Scagnoli S, Sirgiovanni G, et al. Adjuvant chemotherapy in resected colon cancer: When, how and how long? Surg Oncol Elsevier. 2019;30:100–7.
Chan GHJ, Chee CE. Making sense of adjuvant chemotherapy in colorectal cancer. J Gastrointest Oncol. 2019;10:1183–92.
McKeown E, Nelson DW, Johnson EK, Maykel JA, Stojadinovic A, Nissan A, et al. Current approaches and challenges for monitoring treatment response in colon and rectal cancer. J Cancer. 2014;5:31–43.
Kotelevets L, Chastre E, Desmaële D, Couvreur P. Nanotechnologies for the treatment of colon cancer: from old drugs to new hope. Int J Pharm. 2016;514:24–40.
Van Der Jeught K, Xu HC, Li YJ, Lu XB, Ji G. Drug resistance and new therapies in colorectal cancer. World J Gastroenterol. 2018;24:3834–48.
Ott I, Gust R. Non platinum metal complexes as anti-cancer drugs. Arch Pharm (Weinheim). 2007;340:117–26.
Martín J, Alés MR, Asuero GA. An overview on ligands of therapeutically interest. Pharm Pharmacol Int J. 2018;6:198–214.
Brenner H, Kloor M, Pox CP. Colorectal cancer Lancet England. 2014;383:1490–502.
National Cancer Institute. Drugs approved for colon and rectal cancer [Internet]. 2019 [cited 2020 Aug 2]. Available from: https://www.cancer.gov/about-cancer/treatment/drugs/colorectal.
Wu C. Systemic Therapy for colon cancer. Surg Oncol Clin N Am. Elsevier Inc; 2018;27:235–42.
Culy CR, Clemett D, Wiseman LR. Oxaliplatin drugs. 2000;60:895–924.
Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14:1208–15.
Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371:1007–16.
Cremolini C, Loupakis F, Antoniotti C, Lupi C, Sensi E, Lonardi S, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol England. 2015;16:1306–15.
Mir M, Ahmed N, Rehman AU. Recent applications of PLGA based nanostructures in drug delivery. Colloids Surf B Biointerfaces. 2017;159:217–31.
Pinho JO, Matias M, Gaspar MM. Emergent nanotechnological strategies for systemic chemotherapy against melanoma. Nanomaterials. 2019;9:1455.
de Almeida A, Oliveira BL, Correia JDG, Soveral G, Casini A. Emerging protein targets for metal-based pharmaceutical agents: an update. Coord Chem Rev. 2013;257:2689–704.
Sullivan MP, Holtkamp HU, Hartinger CG. Antitumor Metallodrugs that Target Proteins. In: Sigel A, Sigel H, Freisinger E, Sigel RKO, editors. Met Dev Action Anticancer Agents. Berlin: De Gruyter; 2018. p. 351–86.
Ghosh S. Cisplatin: the first metal based anticancer drug. Bioorg Chem. 2019;88:102925.
Wong E, Giandornenico CM. Current status of platinum-based antitumor drugs. Chem Rev. 1999;99:2451–66.
Ho YP, Au-Yeung SCF, To KKW. Platinum-based anticancer agents: Innovative design strategies and biological perspectives. Med Res Rev. 2003;23:633–55.
Frezza M, Hindo S, Chen D, Davenport A, Schmitt S, Tomco D, et al. Novel metals and metal complexes as platforms for cancer therapy. Curr Pharm Des. 2010;16:1813–25.
Ndagi U, Mhlongo N, Soliman M. Metal complexes in cancer therapy - an update from drug design perspective. Drug Des Devel Ther. 2017;11:599–616.
Casini A, Sun RWY, Ott I. Medicinal chemistry of gold anticancer metallodrugs. In: Sigel A, Sigel H, Freisinger E, Sigel R, editors. Met Dev Action Anticancer Agents. Berlin, Boston: De Gruyter; 2018. p. 199–217.
Scheffler H, You Y, Ott I. Comparative studies on the cytotoxicity, cellular and nuclear uptake of a series of chloro gold(I) phosphine complexes. Polyhedron. 2010;29:66–9.
Tong KC, Lok CN, Wan PK, Hu D, Fung YME, Chang XY, et al. An anticancer gold(III)-activated porphyrin scaffold that covalently modifies protein cysteine thiols. Proc Natl Acad Sci. 2020;117:1321–9.
Miranda S, Vergara E, Mohr F, de Vos D, Cerrada E, Mendía A, et al. Synthesis, characterization, and in vitro cytotoxicity of some gold(I) and trans platinum(II) thionate complexes containing water-soluble PTA and DAPTA ligands. X-ray crystal structures of [Au(SC4H3N2)(PTA)], trans-[Pt(SC4H3N2)2(PTA)2], trans-[Pt(SC5H4N)2. Inorg Chem Am Chem Soc. 2008;47:5641–8.
Gandin V, Fernandes AP, Rigobello MP, Dani B, Sorrentino F, Tisato F, et al. Cancer cell death induced by phosphine gold(I) compounds targeting thioredoxin reductase. Biochem Pharmacol. 2010;79:90–101.
Santini C, Pellei M, Gandin V, Porchia M, Tisato F, Marzano C. Advances in copper complexes as anticancer agents. Chem Rev. 2014;114:815–62.
Montagner D, Fresch B, Browne K, Gandin V, Erxleben A. A Cu(ii) complex targeting the translocator protein: in vitro and in vivo antitumor potential and mechanistic insights. Chem Commun. 2017;53:134–7.
Leite SMG, Lima LMP, Gama S, Mendes F, Orio M, Bento I, et al. Copper(II) complexes of phenanthroline and histidine containing ligands: synthesis, characterization and evaluation of their DNA cleavage and cytotoxic activity. Inorg Chem. 2016;55:11801–14.
Nunes CJ, Otake AH, Bustos SO, Fazzi RB, Chammas R, Da Costa Ferreira AM. Unlike reactivity of mono- and binuclear imine-copper(II) complexes toward melanoma cells via a tyrosinase-dependent mechanism. Chem Biol Interact. 2019;311:108789.
Bacher F, Wittmann C, Nové M, Spengler G, Marć MA, Enyedy EA, et al. Novel latonduine derived proligands and their copper(II) complexes show cytotoxicity in the nanomolar range in human colon adenocarcinoma cells and: in vitro cancer selectivity. Dalt Trans. 2019;48:10464–78.
Gandin V, Pellei M, Tisato F, Porchia M, Santini C, Marzano C. A novel copper complex induces paraptosis in colon cancer cellsviathe activation of ER stress signalling. J Cell Mol Med. 2012;16:142–51.
Pinho JO, Amaral JD, Castro RE, Rodrigues CMP, Casini A, Soveral G, et al. Copper complex nanoformulations featuring highly promising therapeutic potential in murine melanoma models. Nanomedicine. 2019;14:835–50.
Nave M, Castro RE, Rodrigues CMP, Casini A, Soveral G, Gaspar MM. Nanoformulations of a potent copper-based aquaporin inhibitor with cytotoxic effect against cancer cells. Nanomedicine. 2016;11:1817–30.
Köpf-Maier P, Köpf H, Neuse EW. Ferricenium complexes: a new type of water-soluble antitumor agent. J Cancer Res Clin Oncol. 1984;108:336–40.
Estrada-Montaño AS, Ryabov AD, Gries A, Gaiddon C, Le Lagadec R. Iron(III) pincer complexes as a strategy for anticancer studies. Eur J Inorg Chem. 2017;2017:1673–8.
Florindo PR, Pereira DM, Borralho PM, Rodrigues CMP, Piedade MFM, Fernandes AC. Cyclopentadienyl–ruthenium(II) and iron(II) organometallic compounds with carbohydrate derivative ligands as good colorectal anticancer agents. J Med Chem. 2015;58:4339–47.
Şen B, Kalhan HK, Demir V, Güler EE, Kayalı HA, Subaşı E. Crystal structures, spectroscopic properties of new cobalt(II), nickel(II), zinc(II) and palladium(II) complexes derived from 2-acetyl-5-chloro thiophene thiosemicarbazone: anticancer evaluation. Mater Sci Eng C. 2019;98:550–9.
Pessoa JC, Etcheverry S, Gambino D. Vanadium compounds in medicine. Coord Chem Rev. 2015;301–302:24–48.
Kioseoglou E, Petanidis S, Gabriel C, Salifoglou A. The chemistry and biology of vanadium compounds in cancer therapeutics. Coord Chem Rev. 2015;301–302:87–105.
León IE, Cadavid-Vargas JF, Tiscornia I, Porro V, Castelli S, Katkar P, et al. Oxidovanadium(IV) complexes with chrysin and silibinin: anticancer activity and mechanisms of action in a human colon adenocarcinoma model. J Biol Inorg Chem. 2015;20:1175–91.
Kowalski S, Wyrzykowski D, Inkielewicz-Stępniak I. Molecular and cellular mechanisms of cytotoxic activity of vanadium compounds against cancer cells. Molecules. 2020;25:1757.
Reytman L, Hochman J, Tshuva EY. Anticancer diaminotris(phenolato) vanadium(V) complexes: ligand-metal interplay. J Coord Chem. 2018;71:2003–11.
Rana SR, McCaffrey V, Rabquer BJ. Vanadium complex induced cancer cell death via RIPK3 activated necroptosis. FASEB J. 2018;31:876.6.
Sinha A, Banerjee K, Banerjee A, Sarkar A, Ahir M, Adhikary A, et al. Induction of apoptosis in human colorectal cancer cell line, HCT-116 by a vanadium- Schiff base complex. Biomed Pharmacother. 2017;92:509–18.
Sanna D, Ugone V, Micera G, Buglyó P, Bíró L, Garribba E. Speciation in human blood of Metvan, a vanadium based potential anti-tumor drug. Dalt Trans. 2017;46:8950–67.
León IE, Ruiz MC, Franca CA, Parajón-Costa BS, Baran EJ. Metvan, bis(4,7-Dimethyl-1,10-phenanthroline)sulfatooxidovanadium(IV): DFT and spectroscopic study—antitumor action on human bone and colorectal cancer cell lines. Biol Trace Elem Res. 2019;191:81–7.
Sinha A, Banerjee K, Banerjee A, Das S, Choudhuri SK. Synthesis, characterization and biological evaluation of a novel vanadium complex as a possible anticancer agent. J Organomet Chem. 2014;772–773:34–41.
Bratsos I, Gianferrara T, Alessio E, Hartinger CG, Jakupec MA, Keppler BK. Ruthenium and other non-platinum anticancer compounds. In: Alessio E, editor. Bioinorg Med Chem. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA; 2011. p. 151–74.
Lazarević T, Rilak A, Bugarčić ŽD. Platinum, palladium, gold and ruthenium complexes as anticancer agents: current clinical uses, cytotoxicity studies and future perspectives. Eur J Med Chem. 2017;142:8–31.
Carter R, Westhorpe A, Romero M, Habtemariam A, Gallevo C, Bark Y, et al. Radiosensitisation of human colorectal cancer cells by ruthenium(II) arene anticancer complexes. Sci Rep. 2016;6:20596.
Ma DL, Wu C, Wu KJ, Leung CH. Iridium(III) complexes targeting apoptotic cell death in cancer cells. Molecules. 2019;24:2739.
Lord RM, Zegke M, Henderson IR, Pask CM, Shepherd HJ, McGowan PC. β-Ketoiminato iridium(III) organometallic complexes: selective cytotoxicity towards colorectal cancer cells HCT116 p53 -/-. Chem - A Eur J. 2019;25:495–500.
Fischer-Fodor E, Mikláš R, Rišiaňová L, Cenariu M, Grosu IG, Virag P, et al. Novel palladium(II) complexes that influence prominin-1/CD133 expression and stem cell factor release in tumor cells. Molecules. 2017;22:561.
Rajpoot K, Jain SK. Colorectal cancer-targeted delivery of oxaliplatin via folic acid-grafted solid lipid nanoparticles: preparation, optimization, and in vitro evaluation. Artif Cells, Nanomedicine, Biotechnol. 2018;46:1236–47.
Mjos KD, Orvig C. Metallodrugs in medicinal inorganic chemistry. Chem Rev. 2014;114:4540–63.
Stewart DJ. Mechanisms of resistance to cisplatin and carboplatin. Crit Rev Oncol Hematol. 2007;63:12–31.
Bayón-Cordero L, Alkorta I, Arana L. Application of solid lipid nanoparticles to improve the efficiency of anticancer drugs. Nanomaterials. 2019;9:474.
Makovec T. Cisplatin and beyond: molecular mechanisms of action and drug resistance development in cancer chemotherapy. Radiol Oncol. 2019;53:148–58.
Komarova NL, Wodarz D. Drug resistance in cancer: principles of emergence and prevention. Proc Natl Acad Sci. 2005;102:9714–9.
Sguizzato M, Cortesi R, Gallerani E, Drechsler M, Marvelli L, Mariani P, et al. Solid lipid nanoparticles for the delivery of 1,3,5-triaza-7-phosphaadamantane (PTA) platinum (II) carboxylates. Mater Sci Eng C. 2017;74:357–64.
Belfiore L, Saunders DN, Ranson M, Thurecht KJ, Storm G, Vine KL. Towards clinical translation of ligand-functionalized liposomes in targeted cancer therapy: challenges and opportunities. J Control Release. 2018;277:1–13.
Sanna V, Pala N, Sechi M. Targeted therapy using nanotechnology: focus on cancer. Int J Nanomedicine. 2014;9:467–83.
Silva CO, Pinho JO, Lopes JM, Almeida AJ, Gaspar MM, Reis C. Current trends in cancer nanotheranostics: metallic, polymeric, and lipid-based systems. Pharmaceutics. 2019;11:22.
Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MDP, Acosta-Torres LS, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology. 2018;16:71.
Griffin BT, Guo J, Presas E, Donovan MD, Alonso MJ, O’Driscoll CM. Pharmacokinetic, pharmacodynamic and biodistribution following oral administration of nanocarriers containing peptide and protein drugs. Adv Drug Deliv Rev. 2016;106:367–80.
Durán-Lobato M, Niu Z, Alonso MJ. Oral delivery of biologics for precision medicine. Adv Mater. 2020;32:1901935.
Attia MF, Anton N, Wallyn J, Omran Z, Vandamme TF. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J Pharm Pharmacol. 2019;71:1185–98.
Mishra B, Chaurasia S. Design of novel chemotherapeutic delivery systems for colon cancer therapy based on oral polymeric nanoparticles. Ther Deliv. 2017;8:29–47.
Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65:271–84.
Banerjee A, Pathak S, Subramanium VD, Dharanivasan G, Murugesan R, Verma RS. Strategies for targeted drug delivery in treatment of colon cancer: current trends and future perspectives. Drug Discov Today. 2017;22:1224–32.
Gaspar MM, Radomska A, Gobbo OL, Bakowsky U, Radomski MW, Ehrhardt C. Targeted delivery of transferrin-conjugated liposomes to an orthotopic model of lung cancer in nude rats. J Aerosol Med Pulm Drug Deliv. 2012;25:310–8.
Song H, Su C, Cui W, Zhu B, Liu L, Chen Z, et al. Folic acid-chitosan conjugated nanoparticles for improving tumor-targeted drug delivery. Biomed Res Int. 2013;2013:1–6.
Sharma M, Malik R, Verma A, Dwivedi P, Banoth GS, Pandey N, et al. Folic acid conjugated guar gum nanoparticles for targeting methotrexate to colon cancer. J Biomed Nanotechnol. 2013;9:96–106.
Santos-Rebelo A, Kumar P, Pillay V, Choonara YE, Eleutério C, Figueira M, et al. Development and mechanistic insight into the enhanced cytotoxic potential of parvifloron D albumin nanoparticles in EGFR-overexpressing pancreatic cancer cells. Cancers (Basel). 2019;11:1733.
Elechalawar CK, Sridharan K, Pal A, Ahmed MT, Yousuf M, Adhikari SS, et al. Cationic folate-mediated liposomal delivery of bis-arylidene oxindole induces efficient melanoma tumor regression. Biomater Sci. 2017;5:1898–909.
Fernandez-Fernandez A, Manchanda R, McGoron AJ. Theranostic applications of nanomaterials in cancer: drug delivery, image-guided therapy, and multifunctional platforms. Appl Biochem Biotechnol. 2011;165:1628–51.
Bulbake U, Doppalapudi S, Kommineni N, Khan W. Liposomal formulations in clinical use: an updated review. Pharmaceutics. 2017;9:12.
Lamichhane N, Udayakumar T, D’Souza W, Simone C II, Raghavan S, Polf J, et al. Liposomes: clinical applications and potential for image-guided drug delivery. Molecules. 2018;23:288.
Kennedy PJ, Sousa F, Ferreira D, Pereira C, Nestor M, Oliveira C, et al. Fab-conjugated PLGA nanoparticles effectively target cancer cells expressing human CD44v6. Acta Biomater. 2018;81:208–18.
Csaba N, Caamaño P, Sánchez A, Domínguez F, Alonso MJ. PLGA: poloxamer and PLGA: poloxamine blend nanoparticles: new carriers for gene delivery. Biomacromol. 2005;6:271–8.
Cadete A, Alonso MJ. Targeting cancer with hyaluronic acid-based nanocarriers: recent advances and translational perspectives. Nanomedicine. 2016;11:2341–57.
Cruz MEM, Simões SI, Corvo ML, Martins MBF, Gaspar MM. Formulation of NPDDS for macromolecules. In: Pathak Y, Thassu D, editors. Drug Deliv Nanoparticles Formul Charact. New York, USA: Informa Healthcare; 2009. p. 35–49.
Yang C, Merlin D. Lipid-based drug delivery nanoplatforms for colorectal cancer therapy. Nanomaterials. 2020;10:1424.
Bozzuto G, Molinari A. Liposomes as nanomedical devices. Int J Nanomedicine. 2015;10:975–99.
Perrie Y, Ramsay E. Nanomedicines: exploring the past, present and future. Drug Discov World. 2017;18:17–22.
Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303:1818–22.
Gabizon AA. Stealth liposomes and tumor targeting: one step further in the quest for the magic bullet. Clin Cancer Res. 2001;7:223–5.
Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev. 2013;65:36–48.
Deshpande S, Sharma S, Koul V, Singh N. Core-shell nanoparticles as an efficient, sustained, and triggered drug-delivery system. ACS Omega. 2017;2:6455–63.
Lila ASA, Ishida T. Liposomal delivery systems: design optimization and current applications. Biol Pharm Bull. 2017;40:1–10.
Simões S, Nuno Moreira J, Fonseca C, Düzgüneş Pedroso N, De Lima MC. On the formulation of pH-sensitive liposomes with long circulation times. Adv Drug Deliv Rev. 2004;56:947–65.
Fan Y, Chen C, Huang Y, Zhang F, Lin G. Study of the pH-sensitive mechanism of tumor-targeting liposomes. Colloids Surfaces B Biointerfaces. 2017;151:19–25.
Kamps J, Koning G, Velinova M, Morselt H, Wilkens M, Gorter A, et al. Uptake of long-circulating immunoliposomes, directed against colon adenocarcinoma cells, by liver metastases of colon cancer. J Drug Target. 2000;8:235–45.
Zalba S, Contreras AM, Haeri A, ten Hagen TLM, Navarro I, Koning G, et al. Cetuximab-oxaliplatin-liposomes for epidermal growth factor receptor targeted chemotherapy of colorectal cancer. J Control Release. 2015;210:26–38.
Zhang B, Wang T, Yang S, Xiao Y, Song Y, Zhang N, et al. Development and evaluation of oxaliplatin and irinotecan co-loaded liposomes for enhanced colorectal cancer therapy. J Control Release. 2016;238:10–21.
Bansal D, Gulbake A, Tiwari J, Jain SK. Development of liposomes entrapped in alginate beads for the treatment of colorectal cancer. Int J Biol Macromol. 2016;82:687–95.
Stathopoulos GP, Boulikas T. Lipoplatin formulation review article. J Drug Deliv. 2012;2012:1–10.
Jehn CF, Boulikas T, Kourvetaris A, Possinger K, Lüftner D. Pharmacokinetics of liposomal cisplatin (lipoplatin) in combination with 5-FU in patients with advanced head and neck cancer: first results of a phase III study. Anticancer Res. 2007;27:471–5.
Kosmas C, Angel J, Athanasiou A, Rapti A, Karanikas C, Lambaki S, et al. 9088 Phase III study of lipoplatin plus gemcitabine versus cisplatin plus gemcitabine in advanced NSCLC; interim analysis. Eur J Cancer Suppl. 2009;7:531.
Dragovich T, Mendelson D, Kurtin S, Richardson K, Von Hoff D, Hoos A. A phase 2 trial of the liposomal DACH platinum L-NDDP in patients with therapy-refractory advanced colorectal cancer. Cancer Chemother Pharmacol. 2006;58:759–64.
Hang Z, Cooper MA, Ziora ZM. Platinum-based anticancer drugs encapsulated liposome and polymeric micelle formulation in clinical trials. Biochem Compd. 2016;4:1.
Seetharamu N, Kim E, Hochster H, Martin F, Muggia F. Phase II study of liposomal cisplatin (SPI-77) in platinum-sensitive recurrences of ovarian cancer. Anticancer Res. 2010;30:541–6.
Kim ES, Lu C, Khuri FR, Tonda M, Glisson BS, Liu D, et al. A phase II study of STEALTH cisplatin (SPI-77) in patients with advanced non-small cell lung cancer. Lung Cancer. 2001;34:427–32.
de Jonge MJA, Slingerland M, Loos WJ, Wiemer EAC, Burger H, Mathijssen RHJ, et al. Early cessation of the clinical development of LiPlaCis, a liposomal cisplatin formulation. Eur J Cancer. 2010;46:3016–21.
U.S. National Health Institute. Phase I/II Study to evaluate the safety and tolerability of LiPlaCis in patients with advanced or refractory tumours (LiPlaCis) [Internet]. 2019 [cited 2020 Sep 10]. Available from: https://clinicaltrials.gov/ct2/show/NCT01861496.
Stathopoulos GP, Boulikas T, Kourvetaris A, Stathopoulos J. Liposomal oxaliplatin in the treatment of advanced cancer: a phase I study. Anticancer Res. 2006;26:1489–93.
Geszke-Moritz M, Moritz M. Solid lipid nanoparticles as attractive drug vehicles: composition, properties and therapeutic strategies. Mater Sci Eng C. 2016;68:982–94.
Mei L, Zhang Z, Zhao L, Huang L, Yang XL, Tang J, et al. Pharmaceutical nanotechnology for oral delivery of anticancer drugs. Adv Drug Deliv Rev. 2013;65:880–90.
Muller RH, Shegokar R, Keck CM. 20 years of lipid nanoparticles (SLN & NLC): present state of development & industrial applications. Curr Drug Discov Technol. 2011;8:207–27.
Gaspar DP, Gaspar MM, Eleutério CV, Grenha A, Blanco M, Gonçalves LMD, et al. Microencapsulated solid lipid nanoparticles as a hybrid platform for pulmonary antibiotic delivery. Mol Pharm. 2017;14:2977–90.
Madan J, Pandey RS, Jain V, Katare OP, Chandra R, Katyal A. Poly (ethylene)-glycol conjugated solid lipid nanoparticles of noscapine improve biological half-life, brain delivery and efficacy in glioblastoma cells. Nanomed Nanotechnol, Biol Med. 2013;9:492–503.
Wissing SA, Kayser O, Müller RH. Solid lipid nanoparticles for parenteral drug delivery. Adv Drug Deliv Rev. 2004;56:1257–72.
Tummala S, Gowthamarajan K, Satish Kumar M, Praveen T, Yamjala K, Tripuraneni NS, et al. Formulation and optimization of oxaliplatin immuno-nanoparticles using Box-Behnken design and cytotoxicity assessment for synergistic and receptor-mediated targeting in the treatment of colorectal cancer. Artif Cells, Nanomedicine, Biotechnol. 2016;44:1835–50.
Guo J, Yu Z, Das M, Huang L. Nano codelivery of oxaliplatin and folinic acid achieves synergistic chemo-immunotherapy with 5-fluorouracil for colorectal cancer and liver metastasis. ACS Nano. 2020;14:5075–89.
Kotelevets L, Chastre E, Caron J, Mougin J, Bastian G, Pineau A, et al. A squalene-based nanomedicine for oral treatment of colon cancer. Cancer Res. 2017;77:2964–75.
George A, Shah PA, Shrivastav PS. Natural biodegradable polymers based nano-formulations for drug delivery: a review. Int J Pharm. 2019;561:244–64.
Ahlawat J, Henriquez G, Narayan M. Enhancing the delivery of chemotherapeutics: role of biodegradable polymeric nanoparticles. Molecules. 2018;23:1–20.
Nowotnik DP. AP5346 (ProLindacTM), A DACH platinum polymer conjugate in phase II trials against ovarian cancer. Curr Bioact Compd. 2011;7:21–6.
Mochida Y, Cabral H, Kataoka K. Polymeric micelles for targeted tumor therapy of platinum anticancer drugs. Expert Opin Drug Deliv. 2017;14:1423–38.
Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Préat V. PLGA-based nanoparticles: an overview of biomedical applications. J Control Release. 2012;161:505–22.
Acharya S, Sahoo SK. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv Drug Deliv Rev. 2011;63:170–83.
Mirakabad FST, Nejati-Koshki K, Akbarzadeh A, Yamchi MR, Milani M, Zarghami N, et al. PLGA-based nanoparticles as cancer drug delivery systems. Asian Pacific J Cancer Prev. 2014;15:517–35.
Matoba T, Egashira K. Nanoparticle-mediated drug delivery system for cardiovascular disease. Int Heart J. 2014;55:281–6.
Chaudhary Z, Ahmed N, Rehman AU, Khan GM. Lipid polymer hybrid carrier systems for cancer targeting: A review. Int J Polym Mater Polym Biomater. 2018;67:86–100.
Sousa AR, Oliveira MJ, Sarmento B. Impact of CEA-targeting nanoparticles for drug delivery in colorectal cancer. J Pharmacol Exp Ther. 2019;370:657–70.
Mattheolabakis G, Taoufik E, Haralambous S, Roberts ML, Avgoustakis K. In vivo investigation of tolerance and antitumor activity of cisplatin-loaded PLGA-mPEG nanoparticles. Eur J Pharm Biopharm. 2009;71:190–5.
de S. L. Oliveira ALC, de Araújo Júnior RF, de Carvalho TG, Chan AB, Schomann T, Tamburini F, et al. Effect of oxaliplatin-loaded poly (d,l-lactide-co-glycolic acid) (PLGA) nanoparticles combined with retinoic acid and cholesterol on apoptosis, drug resistance, and metastasis factors of colorectal cancer. Pharmaceutics. 2020;12:193.
Margiotta N, Marzano C, Gandin V, Osella D, Ravera M, Gabano E, et al. Revisiting [PtCl2(cis -1,4-DACH)]: an underestimated antitumor drug with potential application to the treatment of oxaliplatin-refractory colorectal cancer. J Med Chem. 2012;55:7182–92.
Margiotta N, Savino S, Denora N, Marzano C, Laquintana V, Cutrignelli A, et al. Encapsulation of lipophilic kiteplatin Pt(IV) prodrugs in PLGA-PEG micelles. Dalt Trans. 2016;45:13070–81.
de Moraes PD, Pessine FBT. Formulation of functionalized PLGA nanoparticles with folic acid-conjugated chitosan for carboplatin encapsulation. Eur Polym J. 2018;108:311–21.
Hackl CM, Schoenhacker-Alte B, Klose MHM, Henke H, Legina MS, Jakupec MA, et al. Synthesis and in vivo anticancer evaluation of poly(organo)phosphazene-based metallodrug conjugates. Dalt Trans. 2017;46:12114–24.
Izadi Z, Divsalar A, Saboury AA, Sawyer L. β-Lactoglobulin-pectin nanoparticle-based oral drug delivery system for potential treatment of colon cancer. Chem Biol Drug Des. 2016;88:209–16.
Jain A, Jain SK, Ganesh N, Barve J, Beg AM. Design and development of ligand-appended polysaccharidic nanoparticles for the delivery of oxaliplatin in colorectal cancer. Nanomed Nanotechnol, Biol Med. 2010;6:179–90.
Saber MM, Al-mahallawi AM, Nassar NN, Stork B, Shouman SA. Targeting colorectal cancer cell metabolism through development of cisplatin and metformin nano-cubosomes. BMC Cancer. 2018;18:822.
Nishiyama N, Okazaki S, Cabral H, Miyamoto M, Kato Y, Sugiyama Y, et al. Novel cisplatin-incorporated polymeric micelles can eradicate solid tumors in mice. Cancer Res. 2003;63:8977–83.
Wu H, Cabral H, Toh K, Mi P, Chen Y-C, Matsumoto Y, et al. Polymeric micelles loaded with platinum anticancer drugs target preangiogenic micrometastatic niches associated with inflammation. J Control Release. 2014;189:1–10.
Pöthig A, Casini A. Recent developments of supramolecular metal-based structures for applications in cancer therapy and imaging. Theranostics. 2019;9:3150–69.
Woods B, Wenzel MN, Williams T, Thomas SR, Jenkins RL, Casini A. Exo-functionalized metallacages as host-guest systems for the anticancer drug cisplatin. Front Chem. 2019;7:68.
Han J, Räder AFB, Reichart F, Aikman B, Wenzel MN, Woods B, et al. Bioconjugation of supramolecular metallacages to integrin ligands for targeted delivery of cisplatin. Bioconjug Chem. 2018;29:3856–65.
Meier-Menches SM, Zappe K, Bileck A, Kreutz D, Tahir A, Cichna-Markl M, et al. Time-dependent shotgun proteomics revealed distinct effects of an organoruthenium prodrug and its activation product on colon carcinoma cells. Metallomics. 2019;11:118–27.
Steel TR, Hartinger CG. Metalloproteomics for molecular target identification of protein-binding anticancer metallodrugs. Metallomics. 2020;12:1627–36.
Anthony EJ, Bolitho EM, Bridgewater HE, Carter OWL, Donnelly JM, Imberti C, et al. Metallodrugs are unique: opportunities and challenges of discovery and development. Chem Sci. 2020;11:12888–917.
Wenzel M, Casini A. Mass spectrometry as a powerful tool to study therapeutic metallodrugs speciation mechanisms: current frontiers and perspectives. Coord Chem Rev. 2017;352:432–60.
Crommelin DJA, van Hoogevest P, Storm G. The role of liposomes in clinical nanomedicine development. What now? Now what? J Control Release. 2020;318:256–63.
Funding
Fundação para a Ciência e a Tecnologia (FCT) for financial support through Projects UIDB/04138/2020, UIDP/04138/2020 and PTDC/MED-QUI/31721/2017, as well as for the PhD fellowship SFRH/BD/117586/2016.
Author information
Authors and Affiliations
Contributions
All authors whose names appear on the submission made substantial contributions to the article. Pedro Farinha—writing and original draft preparation; Pedro Farinha, Jacinta O. Pinho, Mariana Matias and Maria Manuela Gaspar—writing, editing, and critical revision of intellectual content; Pedro Farinha, Jacinta O. Pinho, Mariana Matias, and Maria Manuela Gaspar—conceptualization and supervision. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
No human subjects or animal studies were involved in the manuscript.
Consent for publication
All authors have given their consent for publication.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Farinha, P., Pinho, J.O., Matias, M. et al. Nanomedicines in the treatment of colon cancer: a focus on metallodrugs. Drug Deliv. and Transl. Res. 12, 49–66 (2022). https://doi.org/10.1007/s13346-021-00916-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-021-00916-7