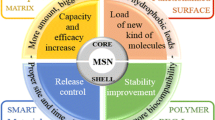
Abstract
The biocompatible nature of mesoporous silica nanoparticles (MSN) attracted researchers’ attention to deliver therapeutic agents in the treatment of various diseases, where their porous nature, high drug loading efficiency, and suitability to functionalize with a specific ligand of MSN helped to obtain the desired outcome. The application of MSN has been extended to deliver small chemicals to large-sized peptides or proteins to fight against complex diseases. Recently, formulation researches with MSN have been progressed for various non-conventional drug delivery systems, including liposome, microsphere, oro-dispersible film, 3D-printed formulation, and microneedle. Low bulk density, retaining mesoporous structure during downstream processing, and lack of sufficient in vivo studies are some of the important issues towards the success of mesoporous silica-based advanced drug delivery systems. The present review has aimed to evaluate the application of MSN in advanced drug delivery systems to critically analyze the role of MSN in the respective formulation over other functionalized polymers. Finally, an outlook on the future direction of MSN-based advanced drug delivery systems has been drawn against the existing challenges with this platform.
Graphical abstract







Similar content being viewed by others
References
Zahedi P, Yoganathan R, Piquette-Miller M, Allen C. Recent advances in drug delivery strategies for treatment of ovarian cancer. Expert Opin Drug Deliv. Taylor & Francis. 2012;9:567–83.
Slowing II, Vivero-Escoto JL, Wu CW, Lin VSY. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv Drug Deliv Rev Elsevier. 2008;60:1278–88.
Mai WX, Meng H. Mesoporous silica nanoparticles: a multifunctional nano therapeutic system. Integr Biol (United Kingdom). Royal Society of Chemistry. 2013;5:19–28.
Choudhury H, Maheshwari R, Pandey M, Tekade M, Gorain B, Tekade RK. Advanced nanoscale carrier-based approaches to overcome biopharmaceutical issues associated with anticancer drug ‘Etoposide.’ Mater Sci Eng C. Elsevier. 2020;106:110275.
Kanaujia P, Poovizhi P, Ng WK, Tan RBH. Amorphous formulations for dissolution and bioavailability enhancement of poorly soluble APIs. Powder Technol. 2015.
Chatterjee B, Hamed Almurisi S, Ahmed Mahdi Dukhan A, Mandal UK, Sengupta P. Controversies with self-emulsifying drug delivery system from pharmacokinetic point of view. Drug Deliv. 2016;23:3639–52.
Karashima M, Sano N, Yamamoto S, Arai Y, Yamamoto K, Amano N, et al. Enhanced pulmonary absorption of poorly soluble itraconazole by micronized cocrystal dry powder formulations. Eur J Pharm Biopharm. Elsevier BV. 2017;115:65–72.
Gorain B, Choudhury H, Biswas E, Barik A, Jaisankar P, Pal TKTK. A novel approach for nanoemulsion components screening and nanoemulsion assay of olmesartan medoxomil through a developed and validated HPLC method. RSC Adv. The Royal Society of Chemistry. 2013;3:10887–93.
Gorain B, Choudhury H, Pandey M, Kesharwani P. Paclitaxel loaded vitamin E-TPGS nanoparticles for cancer therapy. Mater Sci Eng C. 2018;91:868–80.
Shen S-C, Ng W, Onn Chia L, Dong Y-C, Hee Tan R. Applications of mesoporous materials as excipients for innovative drug delivery and formulation. Curr Pharm Des. Bentham Science Publishers Ltd. 2013;19:6270–89.
Bremmell KE, Prestidge CA. Enhancing oral bioavailability of poorly soluble drugs with mesoporous silica based systems: opportunities and challenges. Drug Dev Ind Pharm. Taylor and Francis Ltd. 2019;45:349–58.
Jesus RA, Rabelo AS, Figueiredo RT, Cides Da Silva LC, Codentino IC, Fantini MCA, et al. Synthesis and application of the MCM-41 and SBA-15 as matrices for in vitro efavirenz release study. J Drug Deliv Sci Technol. Editions de Sante. 2016;31:153–9.
Tzankov B, Tzankova V, Aluani D, Yordanov Y, Spassova I, Kovacheva D, et al. Development of MCM-41 mesoporous silica nanoparticles as a platform for pramipexole delivery. J Drug Deliv Sci Technol. Editions de Sante. 2019;51:26–35.
Li Z, Zhang Y, Feng N. Mesoporous silica nanoparticles: synthesis, classification, drug loading, pharmacokinetics, biocompatibility, and application in drug delivery. Expert Opin Drug Deliv. Taylor and Francis Ltd. 2019;16:219–37.
Jia L, Shen J, Li Z, Zhang D, Zhang Q, Liu G, et al. In vitro and in vivo evaluation of paclitaxel-loaded mesoporous silica nanoparticles with three pore sizes. Int J Pharm Int J Pharm. 2013;445:12–9.
Li X, Zhang X, Zhao Y, Sun L. Fabrication of biodegradable Mn-doped mesoporous silica nanoparticles for pH/redox dual response drug delivery. J Inorg Biochem. Elsevier Inc. 2020;202:110887.
Kesse S, Boakye-Yiadom K, Ochete B, Opoku-Damoah Y, Akhtar F, Filli M, et al. Mesoporous silica nanomaterials: versatile nanocarriers for cancer theranostics and drug and gene delivery. Pharmaceutics. MDPI AG. 2019;11:77.
Yan Q, Guo X, Huang X, Meng X, Liu F, Dai P, et al. Gated mesoporous silica nanocarriers for hypoxia-responsive cargo release. ACS Appl Mater Interfaces. American Chemical Society. 2019;11:24377–85.
Shao M, Chang C, Liu Z, Chen K, Zhou Y, Zheng G, et al. Polydopamine coated hollow mesoporous silica nanoparticles as pH-sensitive nanocarriers for overcoming multidrug resistance. Colloids Surfaces B Biointerfaces. Elsevier BV. 2019;183:110427.
Maleki A, Kettiger H, Schoubben A, Rosenholm JM, Ambrogi V, Hamidi M. Mesoporous silica materials: from physico-chemical properties to enhanced dissolution of poorly water-soluble drugs. J Control Release. Elsevier BV. 2017;262:329–47.
Cheng YJ, Luo GF, Zhu JY, Xu XD, Zeng X, Cheng DB, et al. Enzyme-induced and tumor-targeted drug delivery system based on multifunctional mesoporous silica nanoparticles. ACS Appl Mater Interfaces. American Chemical Society. 2015;7:9078–87.
Bagheri E, Ansari L, Abnous K, Taghdisi SM, Charbgoo F, Ramezani M, et al. Silica based hybrid materials for drug delivery and bioimaging. J Control Release. Elsevier BV. 2018;277:57–76.
Bitar A, Ahmad NM, Fessi H, Elaissari A. Silica-based nanoparticles for biomedical applications. Drug Discov Today Elsevier Current Trends. 2012;17:1147–54.
Wang Y, Zhao Q, Han N, Bai L, Li J, Liu J, et al. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomedicine Nanotechnology, Biol Med. Elsevier Inc. 2015;11:313–27.
Slowing II, Trewyn BG, Giri S, Lin VSY. Mesoporous silica nanoparticles for drug delivery and biosensing applications. Adv Funct Mater. John Wiley & Sons, Ltd. 2007;17:1225–36.
Nigro A, Pellegrino M, Greco M, Comandè A, Sisci D, Pasqua L, et al. Dealing with skin and blood-brain barriers: the unconventional challenges of mesoporous silica nanoparticles. Pharmaceutics. MDPI AG. 2018;10:250.
Narayan R, Nayak UY, Raichur AM, Garg S. Mesoporous silica nanoparticles: a comprehensive review on synthesis and recent advances. Pharmaceutics. 2018.
V Chiola, JE Ritsko CV. Process for producing low-bulk density silica. U.S. Patent and Trademark Office. 1971.
Le Page, Madeleine, Raymond Beau, Duchene. J. Porous silica particles containing a crystallized phase and method. U.S. Patent and Trademark Office. 1970.
Yanagisawa T, Shimizu T, Kuroda K, Kato C. The preparation of alkyltriinethylaininonium–kaneinite complexes and their conversion to microporous materials. Bull Chem Soc Jpn. The Chemical Society of Japan. 1990;63:988–92.
Kresge CT, Leonowicz ME, Roth WJ, Vartuli JC, Beck JS. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature. 1992.
Zhao D, Feng J, Huo Q, Melosh N, Fredrickson GH, Chmelka BF, et al. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science. (80). 1998.
Bagshaw SA, Di Renzo F, Fajula F. Preparation of metal-incorporated MSU mesoporous silica molecular sieves. Ti incorporation via a totally non-ionic route. Chem Commun. Royal Society of Chemistry. 1996;2209–10.
Ishikawa T, Matsuda M, Yasukawa A, Kandori K, Inagaki S, Fukushima T, et al. Surface silanol groups of mesoporous silica FSM-16. J Chem Soc - Faraday Trans. 1996;
da Silva F das CM, Costa MJ dos S, da Silva LKR, Batista AM, da Luz GE. Functionalization methods of SBA-15 mesoporous molecular sieve: a brief overview. SN Appl Sci. Springer Science and Business Media LLC. 2019;1:654.
Walstra P. Dispersed systems: basic considerations. Food Chem. 1996. p. 143–6.
Schüth F, Ciesla U, Schacht S, Thieme M, Huo Q, Stucky G. Ordered mesoporous silicas and zirconias: control on length scales between nanometer and micrometer. Mater Res Bull. 1999.
Balkus KJ, Coutinho D, Lucas J, Washmon-Kriel L. Synthesis and characterization of DAM-1 type materials. MRS Online Proc Libr Arch. Cambridge University Press. 2000;628.
Miletto I, Fraccarollo A, Barbero N, Barolo C, Cossi M, Marchese L, et al. Mesoporous silica nanoparticles incorporating squaraine-based photosensitizers: a combined experimental and computational approach. Dalt Trans. 2018.
Abrahamse H, Hamblin MR. New photosensitizers for photodynamic therapy. Biochem J. 2016.
Kempen PJ, Greasley S, Parker KA, Campbell JL, Chang H-Y, Jones JR, et al. Theranostic mesoporous silica nanoparticles biodegrade after pro-survival drug delivery and ultrasound/magnetic resonance imaging of stem cells. Theranostics. Ivyspring International Publisher. 2015;5:631.
Baeza A, Colilla M, Vallet-Regí M. Advances in mesoporous silica nanoparticles for targeted stimuli-responsive drug delivery. Expert Opin. Drug Deliv. 2015.
Möller K, Müller K, Engelke H, Bräuchle C, Wagner E, Bein T. Highly efficient siRNA delivery from core-shell mesoporous silica nanoparticles with multifunctional polymer caps. Nanoscale. 2016.
Martínez-Carmona M, Lozano D, Colilla M, Vallet-Regí M. Lectin-conjugated pH-responsive mesoporous silica nanoparticles for targeted bone cancer treatment. Acta Biomater. 2018.
Vallet-Regi M, Rámila A, Del Real RP, Pérez-Pariente J. A new property of MCM-41: Drug delivery system. Chem Mater. 2001.
Li Z, Barnes JC, Bosoy A, Stoddart JF, Zink JI. Mesoporous silica nanoparticles in biomedical applications. Chem Soc Rev. 2012.
Singh SY, Verma R, Kumar L. Porous oral drug delivery system: tablets. Pharm Chem J. Springer New York LLC. 2018;52:553–61.
Summerlin N, Qu Z, Pujara N, Sheng Y, Jambhrunkar S, McGuckin M, et al. Colloidal mesoporous silica nanoparticles enhance the biological activity of resveratrol. Colloids Surfaces B Biointerfaces. Elsevier BV. 2016;144:1–7.
Zhang Y, Zhi Z, Jiang T, Zhang J, Wang Z, Wang S. Spherical mesoporous silica nanoparticles for loading and release of the poorly water-soluble drug telmisartan. J Control Release J Control Release. 2010;145:257–63.
Wang Z, Chen B, Quan G, Li F, Wu Q, Dian L, et al. Increasing the oral bioavailability of poorly water-soluble carbamazepine using immediate-release pellets supported on SBA-15 mesoporous silica. Int J Nanomedicine. 2012;7:5807–18.
Gangwar RK, Tomar GB, Dhumale VA, Zinjarde S, Sharma RB, Datar S. Curcumin conjugated silica nanoparticles for improving bioavailability and its anticancer applications. J Agric Food Chem. American Chemical Society. 2013;61:9632–7.
He Y, Liang S, Long M, Xu H. Mesoporous silica nanoparticles as potential carriers for enhanced drug solubility of paclitaxel. Mater Sci Eng C Elsevier. 2017;78:12–7.
Takeuchi H, Nagira S, Yamamoto H, Kawashima Y. Solid dispersion particles of amorphous indomethacin with fine porous silica particles by using spray-drying method. Int J Pharm Elsevier. 2005;293:155–64.
Zhang Y, Wang J, Bai X, Jiang T, Zhang Q, Wang S. Mesoporous silica nanoparticles for increasing the oral bioavailability and permeation of poorly water soluble drugs. Mol Pharm Mol Pharm. 2012;9:505–13.
Keasberry NA, Yapp CW, Idris A. Mesoporous silica nanoparticles as a carrier platform for intracellular delivery of nucleic acids. Biochem. Maik Nauka Publishing / Springer SBM. 2017;82:655–62.
Peng H, Dong R, Wang S, Zhang Z, Luo M, Bai C, et al. A pH-responsive nano-carrier with mesoporous silica nanoparticles cores and poly(acrylic acid) shell-layers: fabrication, characterization and properties for controlled release of salidroside. Int J Pharm Elsevier. 2013;446:153–9.
Yamamoto E, Kuroda K. Colloidal mesoporous silica nanoparticles. Bull Chem Soc Jpn. Chemical Society of Japan. 2016;89:501–39.
Lai CY, Trewyn BG, Jeftinija DM, Jeftinija K, Xu S, Jeftinija S, et al. A mesoporous silica nanosphere-based carrier system with chemically removable CdS nanoparticle caps for stimuli-responsive controlled release of neurotransmitters and drug molecules. J Am Chem Soc. 2003.
Thomas MJK, Slipper I, Walunj A, Jain A, Favretto ME, Kallinteri P, et al. Inclusion of poorly soluble drugs in highly ordered mesoporous silica nanoparticles. Int J Pharm Elsevier. 2010;387:272–7.
Lu J, Liong M, Zink JI, Tamanoi F. Mesoporous silica nanoparticles as a delivery system for hydrophobic anticancer drugs. Small. 2007;3:1341–6.
Colilla M, Manzano M, Izquierdo-Barba I, Vallet-Reg M, Boissiére C, Sanchez C. Advanced drug delivery vectors with tailored surface properties made of mesoporous binary oxides submicronic spheres. Chem Mater. 2010.
Wang Y, Sun L, Jiang T, Zhang J, Zhang C, Sun C, et al. The investigation of MCM-48-type and MCM-41-type mesoporous silica as oral solid dispersion carriers for water insoluble cilostazol. Drug Dev Ind Pharm. 2014;40:819–28.
Gao W, Chan JM, Farokhzad OC. PH-responsive nanoparticles for drug delivery. Pharm: Mol; 2010.
Meng H, Xue M, Xia T, Ji Z, Tarn DY, Zink JI, et al. Use of size and a copolymer design feature to improve the biodistribution and the enhanced permeability and retention effect of doxorubicin-loaded mesoporous silica nanoparticles in a murine xenograft tumor model. ACS Nano. 2011.
Tang L, Gabrielson NP, Uckun FM, Fan TM, Cheng J. Size-dependent tumor penetration and in vivo efficacy of monodisperse drug-silica nanoconjugates. Mol Pharm. 2013.
Gary-Bobo M, Mir Y, Rouxel C, Brevet D, Hocine O, Maynadier M, et al. Multifunctionalized mesoporous silica nanoparticles for the in vitro treatment of retinoblastoma: drug delivery, one and two-photon photodynamic therapy. Int J Pharm. 2012;432:99–104.
Singh P, Singh H, Castro-Aceituno V, Ahn S, Kim YJ, Farh MEA, et al. Engineering of mesoporous silica nanoparticles for release of ginsenoside CK and Rh2 to enhance their anticancer and anti-inflammatory efficacy: in vitro studies. J Nanoparticle Res. Springer Netherlands. 2017;19:1–14.
Eivazzadeh-Keihan R, Chenab KK, Taheri-Ledari R, Mosafer J, Hashemi SM, Mokhtarzadeh A, et al. Recent advances in the application of mesoporous silica-based nanomaterials for bone tissue engineering. Mater Sci Eng C. Elsevier Ltd. 2020;107:110267.
Huang C, Zhang Z, Guo Q, Zhang L, Fan F, Qin Y, et al. A dual-model imaging theragnostic system based on mesoporous silica nanoparticles for enhanced cancer phototherapy. Adv Healthc Mater. Adv Healthc Mater. 2019;8:e1900840.
Huang PK, Lin SX, Tsai MJ, Leong MK, Lin SR, Kankala RK, et al. Encapsulation of 16-hydroxycleroda-3,13-dine-16,15-olide in mesoporous silica nanoparticles as a natural dipeptidyl peptidase-4 inhibitor potentiated hypoglycemia in diabetic mice. Nanomaterials. MDPI AG. 2017;7.
Xi C, Zhou J, Du S, Peng S. Autophagy upregulation promotes macrophages to escape mesoporous silica nanoparticle (MSN)-induced NF-κB-dependent inflammation. Inflamm Res Birkhauser Verlag AG. 2016;65:325–41.
Wang Q, Chen C, Liu W, He X, Zhou N, Zhang D, et al. Levofloxacin loaded mesoporous silica microspheres/nanohydroxyapatite/ polyurethane composite scaffold for the treatment of chronic osteomyelitis with bone defects. Sci Rep. Nature Publishing Group. 2017;7.
Brevet D, Gary-Bobo M, Raehm L, Richeter S, Hocine O, Amro K, et al. Mannose-targeted mesoporous silica nanoparticles for photodynamic therapy. Chem Commun. 2009.
Vallet-Regí M, González B, Izquierdo-Barba I. Nanomaterials as promising alternative in the infection treatment. Sci Int J Mol; 2019.
Mora-Raimundo P, Lozano D, Manzano M, Vallet-Regí M. Nanoparticles to knockdown osteoporosis-related gene and promote osteogenic marker expression for osteoporosis treatment. ACS Nano. 2019.
Anglin EJ, Cheng L, Freeman WR, Sailor MJ. Porous silicon in drug delivery devices and materials. Adv Drug Deliv Rev. 2008;60:1266–77.
Tang L, Cheng J. Nonporous silica nanoparticles for nanomedicine application. Nano Today. Elsevier BV. 2013;8:290–312.
Santos HA, Hirvonen J. Nanostructured porous silicon materials: potential candidates for improving drug delivery. Nanomedicine Nanomedicine (Lond). 2012;7:1281–4.
Argyo C, Weiss V, Bräuchle C, Bein T. Multifunctional mesoporous silica nanoparticles as a universal platform for drug delivery. Chem Mater American Chemical Society. 2014;26:435–51.
Kresge CT, Leonowicz ME, Roth WJ, Vartuli JC, Beck JS. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature Nature Publishing Group. 1992;359:710–2.
Grün M, Lauer I, Unger KK. The synthesis of micrometer- and submicrometer-size spheres of ordered mesoporous oxide MCM-41. Adv Mater Wiley-VCH Verlag. 1997;9:254–7.
Unger KK, Kumar D, Grün M, Büchel G, Lüdtke S, Adam T, et al. Synthesis of spherical porous silicas in the micron and submicron size range: challenges and opportunities for miniaturized high-resolution chromatographic and electrokinetic separations. J Chromatogr A Elsevier. 2000;892:47–55.
Freiberg S, Zhu XX. Polymer microspheres for controlled drug release. Int J Pharm Elsevier. 2004;282:1–18.
Noguez Méndez NA, Quirino Barreda CT, Vega AF, Miranda Calderon JE, Urioste CG, Palomec XC, et al. Design and development of pharmaceutical microprocesses in the production of nanomedicine. Nanostructures Oral Med. Elsevier Inc. 2017. p. 669–97.
Sharma N, Purwar N, Gupta PC. Microspheres as drug carriers for controlled drug delivery : a review. Int J Pharm Sci Res. 2015;6:4579–87.
Chouhan R, Goswami S, Bajpai AK. Recent advancements in oral delivery of insulin: from challenges to solutions. Nanostructures Oral Med. Elsevier Inc. 2017. p. 409–33.
Jayant RD, McShane MJ, Srivastava R. Polyelectrolyte-coated alginate microspheres as drug delivery carriers for dexamethasone release coated alginate microspheres for dexamethasone delivery R.D. Jayant et al. Drug Deliv. 2009;16:331–40.
Yamauchi Y, Kuroda K. Rational design of mesoporous metals and related nanomaterials by a soft-template approach. Chem - An Asian J. Chem Asian J. 2008;3:664–76.
Hegazy M, Zhou P, Wu G, Wang L, Rahoui N, Taloub N, et al. Construction of polymer coated core-shell magnetic mesoporous silica nanoparticles with triple responsive drug delivery. Polym Chem. 2017.
Song Y, Yang LY, Wang Y guang, Yu D, Shen J, Ouyang X kun. Highly efficient adsorption of Pb(II) from aqueous solution using amino-functionalized SBA-15/calcium alginate microspheres as adsorbent. Int J Biol Macromol. Elsevier BV. 2019;125:808–19.
Hu L, Sun C, Song A, Chang D, Zheng X, Gao Y, et al. Alginate encapsulated mesoporous silica nanospheres as a sustained drug delivery system for the poorly water-soluble drug indomethacin. Asian J Pharm Sci. Shenyang Pharmaceutical University. 2014;9:183–90.
Liao Y Te, Liu CH, Yu J, Wu KCW. Liver cancer cells: targeting and prolonged-release drug carriers consisting of mesoporous silica nanoparticles and alginate microspheres. Int J Nanomedicine. Dove Medical Press Ltd. 2014;9:2767–78.
Liu C, Guo J, Yang W, Hu J, Wang C, Fu S. Magnetic mesoporous silica microspheres with thermo-sensitive polymer shell for controlled drug release. J Mater Chem. The Royal Society of Chemistry. 2009;19:4764–70.
Chang B, Sha X, Guo J, Jiao Y, Wang C, Yang W. Thermo and pH dual responsive, polymer shell coated, magnetic mesoporous silica nanoparticles for controlled drug release. J Mater Chem. 2011.
Tian Z, Yu X, Ruan Z, Zhu M, Zhu Y, Hanagata N. Magnetic mesoporous silica nanoparticles coated with thermo-responsive copolymer for potential chemo- and magnetic hyperthermia therapy. Microporous Mesoporous Mater. Elsevier BV. 2018;256:1–9.
Shen Y, Tang H, Radosz M, Van Kirk E, Murdoch WJ. PH-responsive nanoparticles for cancer drug delivery. Methods Mol Biol Humana Press. 2008;437:183–216.
Wen H, Guo J, Chang B, Yang W. PH-responsive composite microspheres based on magnetic mesoporous silica nanoparticle for drug delivery. Eur J Pharm Biopharm Elsevier. 2013;84:91–8.
Gandavarapu NR, Azagarsamy MA, Anseth KS. Photo-click living strategy for controlled, reversible exchange of biochemical ligands. Adv Mater Wiley-VCH Verlag. 2014;26:2521–6.
Li X, Hong CY, Pan CY. Preparation and characterization of hyperbranched polymer grafted mesoporous silica nanoparticles via self-condensing atom transfer radical vinyl polymerization. Polymer (Guildf). Elsevier BV. 2010;51:92–9.
Hong CY, Li X, Pan CY. Smart core-shell nanostructure with a mesoporous core and a stimuli-responsive nanoshell synthesized via surface reversible addition-fragmentation chain transfer polymerization. J Phys Chem C. American Chemical Society. 2008;112:15320–4.
Hong CY, You YZ, Pan CY. Synthesis of water-soluble multiwalled carbon nanotubes with grafted temperature-responsive shells by surface RAFT polymerization. Chem Mater American Chemical Society. 2005;17:2247–54.
You YZ, Hong CY, Pan CY. Directly growing ionic polymers on multi-walled carbon nanotubes via surface RAFT polymerization - IOPscience. Nanotechnology. 2006;17:2350.
Stenzel MH, Zhang L, Huck WTS. Temperature-responsive glycopolymer brushes synthesized via RAFT polymerization using the Z-group approach. Macromol Rapid Commun. John Wiley & Sons, Ltd. 2006;27:1121–6.
Narayanaswamy R, Torchilin VP. Hydrogels and their applications in targeted drug delivery. Molecules. MDPI AG. 2019;24:603.
Chen X, Liu Z, Parker SG, Zhang X, Gooding JJ, Ru Y, et al. Light-induced hydrogel based on tumor-targeting mesoporous silica nanoparticles as a theranostic platform for sustained cancer treatment. ACS Appl Mater Interfaces. American Chemical Society. 2016;8:15857–63.
Zhao P, Liu H, Deng H, Xiao L, Qin C, Du Y, et al. A study of chitosan hydrogel with embedded mesoporous silica nanoparticles loaded by ibuprofen as a dual stimuli-responsive drug release system for surface coating of titanium implants. Colloids Surfaces B Biointerfaces Elsevier. 2014;123:657–63.
Zhu M, Zhu Y, Zhang L, Shi J. Preparation of chitosan/mesoporous silica nanoparticle composite hydrogels for sustained co-delivery of biomacromolecules and small chemical drugs. Sci Technol Adv Mater. Taylor & Francis. 2013;14:45005–14.
Rao Z, Liu S, Wu R, Wang G, Sun Z, Bai L, et al. Fabrication of dual network self-healing alginate/guar gum hydrogels based on polydopamine-type microcapsules from mesoporous silica nanoparticles. Int J Biol Macromol. Elsevier BV. 2019;129:916–26.
Karaman D Ṣen, Patrignani G, Rosqvist E, Smått JH, Orłowska A, Mustafa R, et al. Mesoporous silica nanoparticles facilitating the dissolution of poorly soluble drugs in orodispersible films. Eur J Pharm Sci. Elsevier BV. 2018;122:152–9.
LaBauve AE, Rinker TE, Noureddine A, Serda RE, Howe JY, Sherman MB, et al. Lipid-coated mesoporous silica nanoparticles for the delivery of the ML336 antiviral to inhibit encephalitic alphavirus infection. Sci Rep Nature Publishing Group. 2018;8:1–13.
Butler KS, Durfee PN, Theron C, Ashley CE, Carnes EC, Brinker CJ. Protocells: modular mesoporous silica nanoparticle-supported lipid bilayers for drug delivery. Small. 2016;12:2173–85.
Nel AE, Meng H, Xiangsheng L. Mesoporous silica nanoparticles with lipid bilayer coating for cargo delivery [Internet]. 2018. Available from: https://patents.google.com/patent/US10143660B2/en?q=Mesoporous+silica+nanoparticle+lipid+bilayer+coating+cargo+delivery&oq=+Mesoporous+silica+nanoparticle+with+lipid+bilayer+coating+for+cargo+delivery
Wu X, Wang Z, Zhu D, Zong S, Yang L, Zhong Y, et al. PH and thermo dual-stimuli-responsive drug carrier based on mesoporous silica nanoparticles encapsulated in a copolymer-lipid bilayer. ACS Appl Mater Interfaces. American Chemical Society. 2013;5:10895–903.
Sun Q, You Q, Wang J, Liu L, Wang Y, Song Y, et al. Theranostic nanoplatform: Triple-modal imaging-guided synergistic cancer therapy based on liposome-conjugated mesoporous silica nanoparticles. ACS Appl Mater Interfaces. American Chemical Society. 2018;10:1963–75.
Yang S, Song S, Han K, Wu X, Chen L, Hu Y, et al. Characterization, in vitro evaluation and comparative study on the cellular internalization of mesoporous silica nanoparticle-supported lipid bilayers. Microporous Mesoporous Mater. Elsevier BV. 2019;284:212–24.
Zhang X, Li F, Guo S, Chen X, Wang X, Li J, et al. Biofunctionalized polymer-lipid supported mesoporous silica nanoparticles for release of chemotherapeutics in multidrug resistant cancer cells. Biomaterials Elsevier. 2014;35:3650–65.
Liu X, Situ A, Kang Y, Villabroza KR, Liao Y, Chang CH, et al. Irinotecan delivery by lipid-coated mesoporous silica nanoparticles shows improved efficacy and safety over liposomes for pancreatic cancer. ACS Nano American Chemical Society. 2016;10:2702–15.
Tu J, Bussmann J, Du G, Gao Y, Bouwstra JA, Kros A. Lipid bilayer-coated mesoporous silica nanoparticles carrying bovine hemoglobin towards an erythrocyte mimic. Int J Pharm. Elsevier BV. 2018;543:169–78.
Pan J, Wan D, Gong J. PEGylated liposome coated QDs/mesoporous silica core-shell nanoparticles for molecular imaging. Chem Commun. The Royal Society of Chemistry. 2011;47:3442–4.
Meng H, Wang M, Liu H, Liu X, Situ A, Wu B, et al. Use of a lipid-coated mesoporous silica nanoparticle platform for synergistic gemcitabine and paclitaxel delivery to human pancreatic cancer in mice. ACS Nano American Chemical Society. 2015;9:3540–57.
Liu J, Jiang X, Ashley C, Brinker CJ. Electrostatically mediated liposome fusion and lipid exchange with a nanoparticle-supported bilayer for control of surface charge, drug containment, and delivery. J Am Chem Soc. American Chemical Society. 2009;131:7567–9.
Liu J, Stace-Naughton A, Jiang X, Brinker CJ. Porous nanoparticle supported lipid bilayers (protocells) as delivery vehicles. J Am Chem Soc. American Chemical Society. 2009;131:1354–5.
Donnelly RF, Raj Singh TR, Woolfson AD. Microneedle-based drug delivery systems: microfabrication, drug delivery, and safety. Drug Deliv Europe PMC Funders. 2010;17:187–207.
Yang J, Liu X, Fu Y, Song Y. Recent advances of microneedles for biomedical applications: drug delivery and beyond. Acta Pharm Sin B. Chinese Academy of Medical Sciences. 2019;9:469–83.
Du G, Woythe L, van der Maaden K, Leone M, Romeijn S, Kros A, et al. Coated and hollow microneedle-mediated intradermal immunization in mice with diphtheria toxoid loaded mesoporous silica nanoparticles. Pharm Res. Springer New York LLC. 2018;35:189.
Lee CH, Cheng SH, Wang YJ, Chen YC, Chen NT, Souris J, et al. Near-infrared mesoporous silica nanoparticles for optical imaging: characterization and in vivo biodistribution. Adv Funct Mater. John Wiley & Sons, Ltd. 2009;19:215–22.
Lei Q, Qiu WX, Hu JJ, Cao PX, Zhu CH, Cheng H, et al. Multifunctional mesoporous silica nanoparticles with thermal-responsive gatekeeper for NIR light-triggered chemo/photothermal-therapy. Small Wiley-VCH Verlag. 2016;12:4286–98.
Sheng Z, Hu D, Xue M, He M, Gong P, Cai L. Indocyanine green nanoparticles for theranostic applications. Nano-Micro Lett. Springer Science and Business Media LLC. 2013;5:145–50.
Pei P, Yang F, Liu J, Hu H, Du X, Hanagata N, et al. Composite-dissolving microneedle patches for chemotherapy and photothermal therapy in superficial tumor treatment. Biomater Sci Royal Society of Chemistry. 2018;6:1414–23.
Xu B, Jiang G, Yu W, Liu D, Zhang Y, Zhou J, et al. H2O2-responsive mesoporous silica nanoparticles integrated with microneedle patches for the glucose-monitored transdermal delivery of insulin. J Mater Chem B. Royal Society of Chemistry. 2017;5:8200–8.
Zeeshan F, Madheswaran T, Pandey M, Gorain B. Three-dimensional (3-D) printing technology exploited for the fabrication of drug delivery systems. Curr Pharm Des. 2018;24:5019–28.
Pandey M, Choudhury H, Fern JLC, Kee ATK, Kou J, Jing JLJ, et al. 3D printing for oral drug delivery: a new tool to customize drug delivery. Drug Deliv Transl Res Springer. 2020;10:986–1001.
Planchette C, Pichler H, Wimmer-Teubenbacher M, Gruber M, Gruber-Woelfler H, Mohr S, et al. Printing medicines as orodispersible dosage forms: effect of substrate on the printed micro-structure. Int J Pharm Elsevier. 2016;509:518–27.
Genina N, Janßen EM, Breitenbach A, Breitkreutz J, Sandler N. Evaluation of different substrates for inkjet printing of rasagiline mesylate. Eur J Pharm Biopharm. 2013;85:1075–83.
Preis M, Rosenholm JM. Printable nanomedicines: the future of customized drug delivery? Ther Deliv Future Medicine Ltd. 2017;8:721–3.
Boehm RD, Miller PR, Daniels J, Stafslien S, Narayan RJ. Inkjet printing for pharmaceutical applications. Mater Today Elsevier Ltd. 2014;17:247–52.
Wickstrm H, Hilgert E, Nyman JO, Desai D, Karaman DŞ, De Beer T, et al. Inkjet printing of drug-loaded mesoporous silica nanoparticles—a platform for drug development. Molecules. MDPI AG. 2017;22:2020.
Lago M. Inkjet printing of nirogacestat loaded mesoporous silica nanoparticles. [Italy]: Universita Degli Studi Di Padova. 2018.
Rosenholm JM, Sahlgren C, Lindén M. Towards multifunctional, targeted drug delivery systems using mesoporous silica nanoparticles - opportunities & challenges. Nanoscale Nanoscale. 2010;2:1870–83.
Zhao Y, Sun X, Zhang G, Trewyn BG, Slowing II, Lin VSY. Interaction of mesoporous silica nanoparticles with human red blood cell membranes: Size and surface effects. ACS Nano American Chemical Society. 2011;5:1366–75.
Huang X, Zhuang J, Teng X, Li L, Chen D, Yan X, et al. The promotion of human malignant melanoma growth by mesoporous silica nanoparticles through decreased reactive oxygen species. Biomaterials. 2010.
Mamaeva V, Sahlgren C, Lindén M. Mesoporous silica nanoparticles in medicine-recent advances. Rev: Adv. Drug Deliv; 2013.
Vallet-Regí M, Colilla M, Izquierdo-Barba I, Manzano M. Mesoporous silica nanoparticles for drug delivery: current insights. Molecules. Multidisciplinary Digital Publishing Institute. 2018;23:47.
Liu T, Li L, Teng X, Huang X, Liu H, Chen D, et al. Single and repeated dose toxicity of mesoporous hollow silica nanoparticles in intravenously exposed mice. Biomaterials. 2011.
Bukara K, Schueller L, Rosier J, Martens MA, Daems T, Verheyden L, et al. Ordered mesoporous silica to enhance the bioavailability of poorly water-soluble drugs: Proof of concept in man. Eur J Pharm Biopharm. 2016.
Germain M, Caputo F, Metcalfe S, Tosi G, Spring K, Åslund AKO, et al. Delivering the power of nanomedicine to patients today. J Control Release. 2020.
Acknowledgements
The authors wish to acknowledge the support of SPPSPTM, SVKM’s NMIMS, Mumbai (India), in completion of the article.
Author information
Authors and Affiliations
Contributions
All the authors have a significant contribution to the article as mentioned in the following (author-wise). First author: construction of the manuscript and writing of the main body, formatting. Second and third author: overall technical review and editing, scientific input for modification of some major sections. Corresponding author: concept, article construction, section-wise review and technical modification, abstract. All the four authors have contributed to the article. The final submitted version has been reviewed by all authors, and all of them agreed on the submission.
Corresponding author
Ethics declarations
Informed consent
On behalf of the authors, the corresponding author hereby ensures the consent of all authors for submitting the article to Drug Delivery and Translational Research Journal.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Stephen, S., Gorain, B., Choudhury, H. et al. Exploring the role of mesoporous silica nanoparticle in the development of novel drug delivery systems. Drug Deliv. and Transl. Res. 12, 105–123 (2022). https://doi.org/10.1007/s13346-021-00935-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-021-00935-4